A novel process for synthesizing a ketal intermediate of 2-(4'-bromomethyl)phenylpropionic acid
A technology of bromomethyl phenyl and intermediate, which is applied in the field of new technology for synthesizing 2-propionic acid intermediate ketal, can solve the problems of many three wastes, the yield needs to be improved, etc., and achieves high selectivity, high conversion rate, high reaction mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
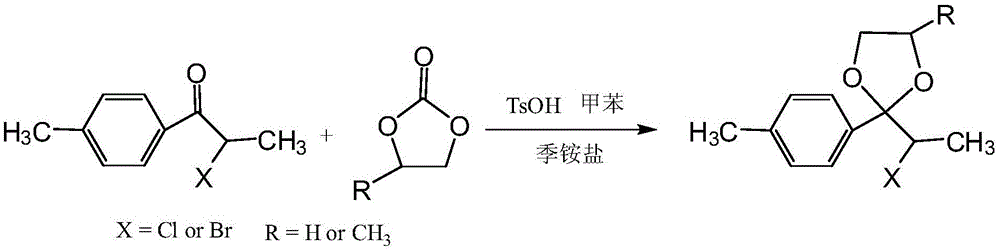
[0021] 18.251g (0.1mol) of 1-p-tolyl-2-chloro-1-propanone, 81.642g (0.8mol) of propylene carbonate, 0.086g (0.0005mol) of anhydrous p-toluenesulfonic acid, 0.032g ( 0.0001mol) of tetrabutylammonium bromide and 150ml of anhydrous toluene are placed in a reaction flask equipped with a water separator, stirred and refluxed for dehydration for 5h, cooled to room temperature, and the ketal yield by liquid chromatography analysis is 63.9% (based on 1-p-tolyl-2-chloro-1-propanone).
Embodiment 2
[0023] 18.252g (0.1mol) of 1-p-tolyl-2-chloro-1-propanone, 12.244g (0.12mol) of propylene carbonate, 3.451g (0.02mol) of anhydrous p-toluenesulfonic acid, 0.085g ( 0.0002mol) of octadecyldimethylbenzyl ammonium chloride and 100ml of anhydrous toluene are placed in a reaction flask equipped with a water separator, stirred and refluxed for dehydration for 42h, cooled to room temperature, and analyzed by liquid chromatography for ketal yield. The yield was 87.3% (calculated as 1-p-tolyl-2-chloro-1-propanone).
Embodiment 3
[0025] 18.248g (0.1mol) of 1-p-tolyl-2-chloro-1-propanone, 20.407g (0.2mol) propylene carbonate, 1.726g (0.01mol) anhydrous p-toluenesulfonic acid, 0.371g ( 0.001mol) of tetrabutylammonium iodide and 150ml of anhydrous toluene, placed in the reaction flask equipped with a water separator, stirred and refluxed for dehydration 20h, cooled to room temperature, liquid chromatography analysis ketal yield 96.4% (with 1 - calculated from p-tolyl-2-chloro-1-propanone).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com