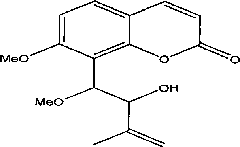
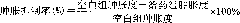Method for synthesizing (+/-)-Murracarpin and anti-inflammatory and analgesic effect thereof
A synthetic method and technology of acetone, applied in the direction of anti-inflammatory agents, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of no anti-inflammatory and analgesic activity, etc., and achieve easy-to-obtain raw materials, simple implementation and operation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (±)-Murracarpin Synthetic Preparation-I
[0042]Step A: Dissolve 8.1 g (50 mmol) of 7-hydroxycoumarin and 20 ml of acetic anhydride in 100 ml of anhydrous acetone, and slowly add 2 ml of pyridine dropwise. After reacting for 1 h at room temperature, a large amount of white precipitate was observed by adding an equal volume of water. The entire reaction system was washed with 10 times the volume of distilled water, and the collected precipitate was 9.73 g (95%) of 7-acetoxycoumarin. used directly in the next reaction.
[0043] Step B: Add 7.5g (37mmol) of 7-acetoxycoumarin to 50ml of trifluoroacetic acid under ice-cooling, and add 7.5g (53.5mmol) of hexamethylenetetramine under vigorous stirring. After cooling to room temperature, start heating to 88°C, and reflux for 8h. Evaporate excess trifluoroacetic acid, add 30ml of distilled water, continue to stir at 60°C for 30min, and a large amount of white precipitate can be seen. Cool to 0°C, collect by filtration and w...
Embodiment 2
[0048] (±)-Murracarpin Synthetic Preparation-II
[0049] Step A: Dissolve 8.1 g (50 mmol) of 7-hydroxycoumarin and 30 ml of acetic anhydride in 120 ml of anhydrous acetone, and slowly add 3 ml of pyridine dropwise. After reacting for 1 h at room temperature, a large amount of white precipitate was observed by adding an equal volume of water. The entire reaction system was washed with 10 times the volume of distilled water, and the collected precipitate was 9.75 g (96%) of 7-acetoxycoumarin. used directly in the next reaction.
[0050] Step B: Add 7.5g (37mmol) of 7-acetoxycoumarin to 40ml of trifluoroacetic acid under ice-cooling, and add 7.5g (53.5mmol) of hexamethylenetetramine under vigorous stirring. After cooling to room temperature, began heating to 90 ° C, reflux 11h. Evaporate excess trifluoroacetic acid, add 30ml of distilled water, continue to stir at 60°C for 30min, and a large amount of white precipitate can be seen. Cool to 0°C, collect by filtration and wash...
Embodiment 3
[0055] Effects of Different Compounds on the Inhibition Rate of Mouse Ear Swelling Caused by Xylene
[0056] Experimental method reference: Chen YF, Tsai HY, Wu TS. Anti-inflammatory and analgesic activities from roots of Angelica pubescens[J].Planta-Med.1995.61(1), 2-8.
[0057] Take (±)-murracarpin, osthole, and umbelliferin, dissolve them in 1% Tween-80 solution, dissolve them by ultrasonic, and prepare them at a concentration of 10 mg / kg. The positive control group was given 0.5mg / kg dexamethasone.
[0058] There were 6 rats in each group, fasted for 18-22 hours before the experiment, without water. The administration group was given each compound by intraperitoneal injection (ig). The positive group ig dexamethasone 0.5mg / kg, the blank control group was given the same volume of normal saline, continuous administration for 7 days, 1 hour after the last administration, 30 μl of xylene was evenly applied to the front and back of the right ear of the mouse, and the left ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com