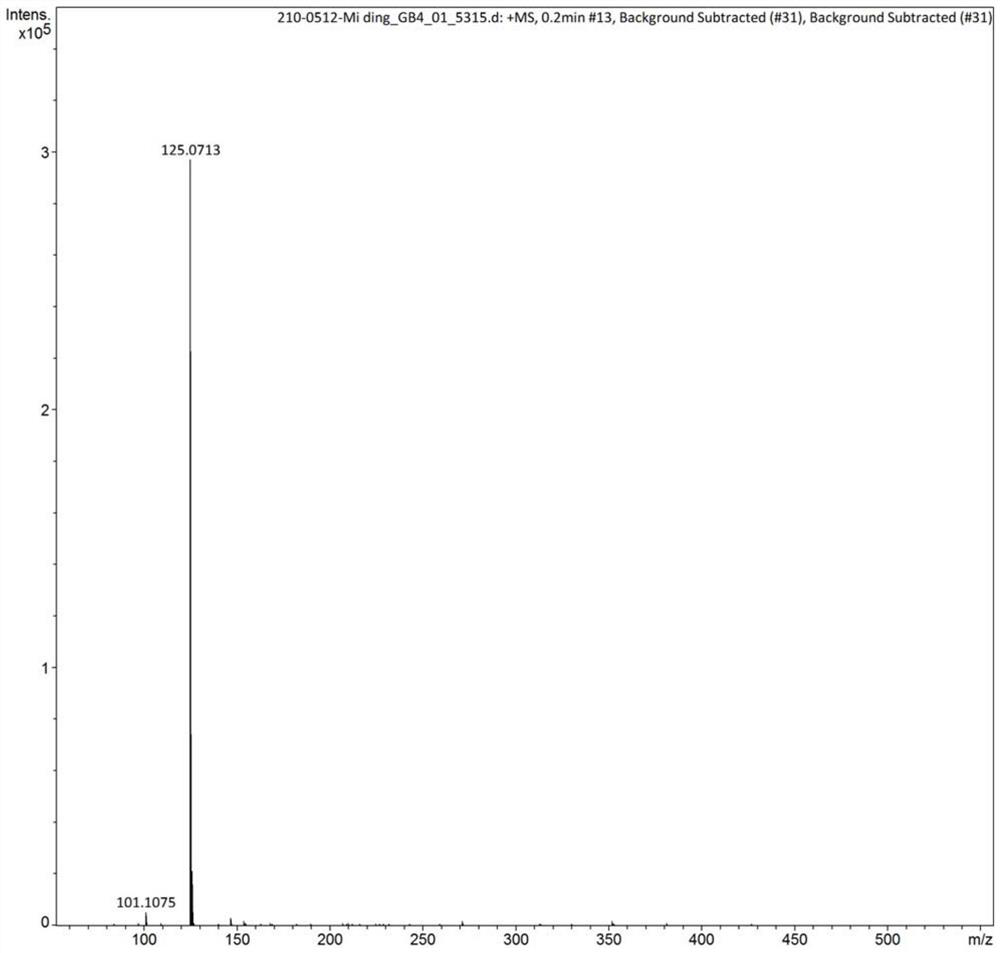
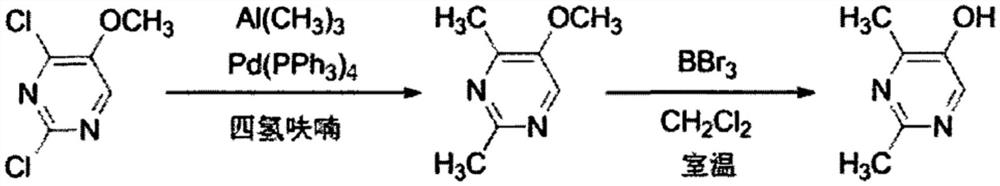
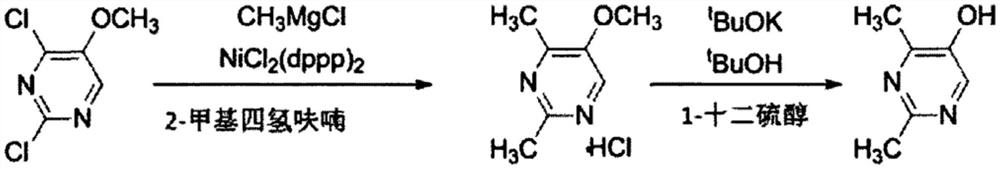
Method for synthesizing 2, 4-dimethyl pyrimidine-5-alcohol serving as intermediate of Leibolifera
A technology of dimethyl pyrimidine and synthesis method, which is applied in the field of organic chemistry, can solve the problems of low EHS risk, achieve the effects of low cost, solve health problems, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Preparation of benzoic acid-2-oxypropyl ester
[0038] Stir the mixture of 20g benzoic acid, 15.9g chloroacetone and 100ml tetrahydrofuran at room temperature, add 45.27g potassium carbonate and 0.2g potassium iodide catalyst to the reaction mixture under temperature control below 40°C, and then stir the resulting mixture at 40°C for reaction 16 hours.
[0039] After the reaction was completed, the reaction mixture was cooled in an ice bath, and then 40 ml of purified water was added dropwise to the reaction mixture. The aqueous phase was separated and washed with 10% aqueous sodium chloride solution. After washing, the organic phase was concentrated until there was no distillate, and 100 ml of n-heptane was added to lower the temperature at 5° C. for crystallization for 0.5 hours, and then the turbid system was filtered. The obtained solid was washed with n-heptane and dried under reduced pressure to obtain 27.1 g of the target compound with a molar yield of 93% an...
Embodiment 2
[0045] 1) Preparation of benzoic acid-2-oxypropyl ester
[0046] Stir the mixture of 100g benzoic acid, 79.5g chloroacetone and 500ml tetrahydrofuran at room temperature, add 226g potassium carbonate and 1g potassium iodide catalyst to the following reaction mixture under temperature control below 40°C, then stir the resulting mixture at 40°C for 16 hours . The reaction mixture was cooled in an ice bath, and then 200 ml of purified water was added dropwise to the reaction mixture. The water phase was separated, and then washed with 10% aqueous sodium chloride solution. After washing, the organic phase was concentrated until there was no distillate, and then 500 ml of n-heptane was added to cool down at 5° C. for crystallization for 0.5 hours, and then the cloudy system was filtered. The obtained solid was washed with n-heptane and dried under reduced pressure to obtain 138.4 g of the target compound with a molar yield of 95% and a purity of 99.5%.
[0047] 2) Preparation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com