2,5-hexanedione synthesis method
A synthetic method, the technology of hexanedione, which is applied in 2 fields, can solve the problems of high cost and rare raw materials, and achieve the effects of easy acquisition, low technical cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
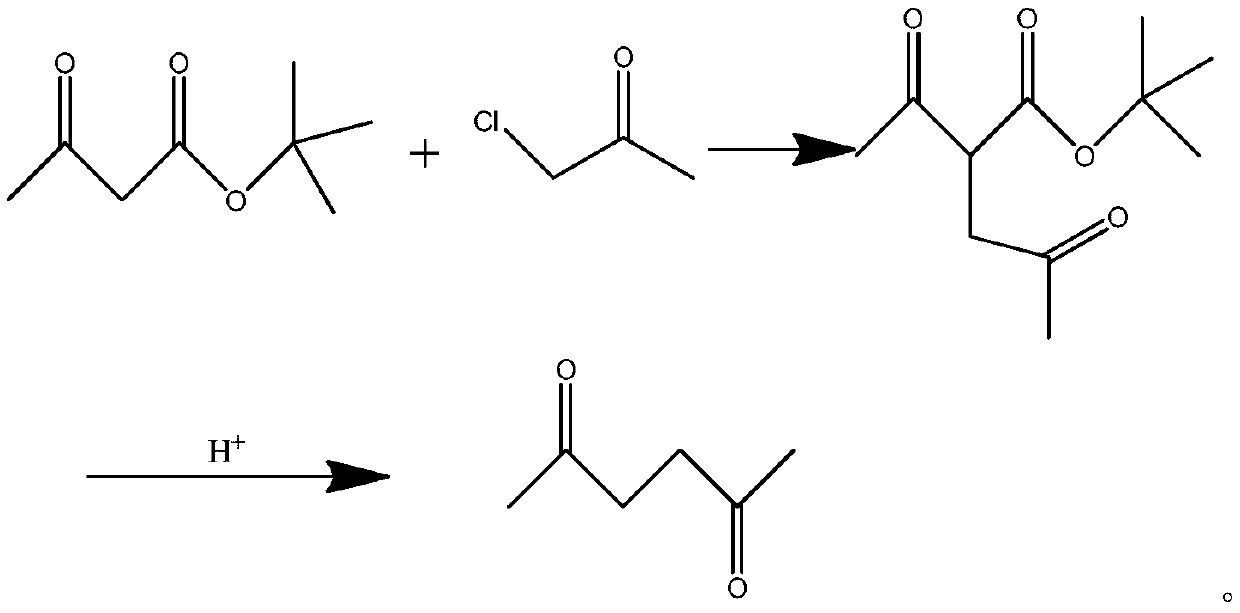
[0011] The present invention provides a kind of synthetic method of 2,5-hexanedione, adopts tert-butyl acetoacetate to react with chloroacetone under the action of alkali to generate tert-butyl 3-acetylmethylacetoacetate, then hydrolyzes under acidic condition, Deacidification gives 2,5-hexanedione,
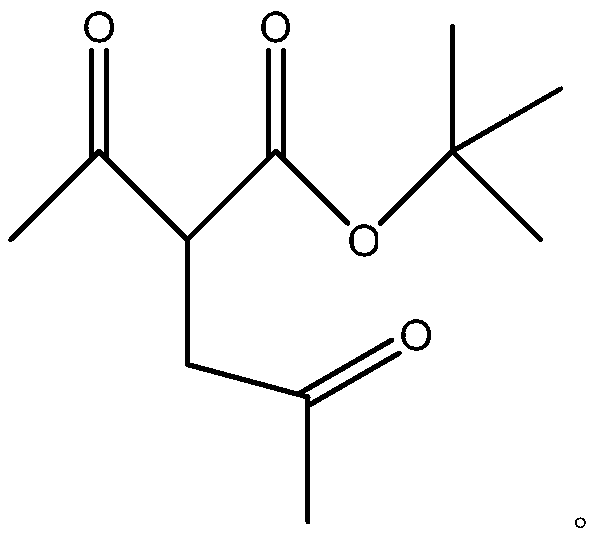
[0012] The synthetic chemical formula of described 2,5-hexanedione is as follows:
[0013]
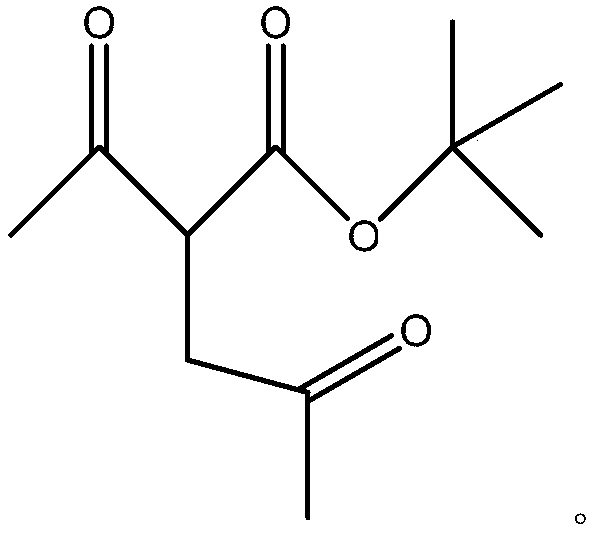
[0014] The general formula of the 2,5-hexanedione intermediate is as follows:
[0015]
[0016] Wherein, the base is selected from anhydrous carbonate, triethylamine, pyridine, and the acid is selected from sulfuric acid, hydrochloric acid, phosphoric acid and trifluoroacetic acid.
Embodiment 1
[0019] In a 2000 ml reactor, add 260 g tert-butyl acetoacetate, 145 g chloroacetone and 1000 g toluene, raise the temperature to 60-80 °C, add 150 kg triethylamine dropwise, and continue the reaction for 5 hours after the drop is complete. Cool, filter, and remove toluene to obtain 290 kg of product tert-butyl 3-acetylmethylacetoacetate with a yield of 90.6%, which is used for the next step reaction.
[0020] Add 500g of 20% sulfuric acid solution and 290g of the product from the previous step into a 1000-liter enamel reaction kettle, heat to 60-70°C for 8 hours, cool, extract with toluene, remove toluene, and distill under reduced pressure to obtain 2,5-hexanedione 138kg, the yield is 90.9%.
Embodiment 2
[0022] In a 2000ml reactor, add 260g of tert-butyl acetoacetate, 145g of chloroacetone and 1000Kg of toluene, raise the temperature to 60-80°C, add 150kg of triethylamine dropwise, continue to react for 8 hours after the addition, cool and filter. Toluene was removed to obtain 280 kg of tert-butyl 3-acetylmethylacetoacetate with a yield of 87.5%. for the next reaction.
[0023] Add 500g of 20% hydrochloric acid solution and 290g of the product from the previous step into a 1000-liter enamel reaction kettle, heat to 60-70°C for 8 hours, cool, extract with toluene, remove toluene, and distill under reduced pressure to obtain 2,5-hexanedione 135kg, the yield is 90.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com