2-methyl-3-hydroxy-6-isopropylquinoline-4-carboxyl acid preparation method
A technology of isopropylquinoline and isopropylaniline, applied in the field of synthesis of organic intermediates, can solve problems such as being difficult to obtain, unsuitable for industrialization, and high corrosiveness of equipment, and achieves improved reaction speed, easy industrialization, and safe operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
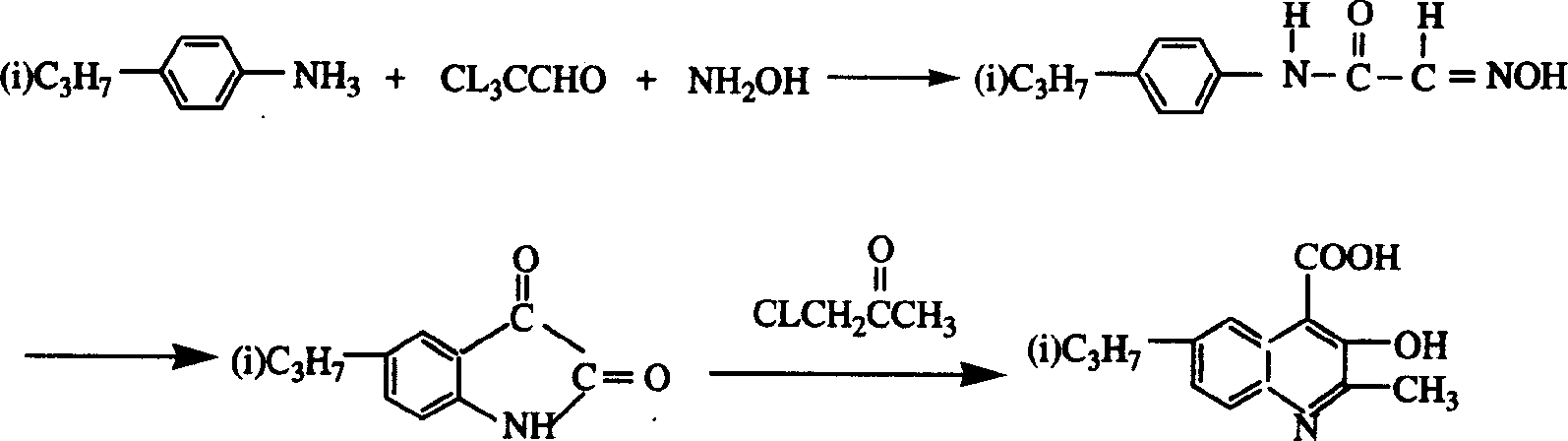
[0020] Add 16.5 grams of chloral hydrate and 142 grams of sodium sulfate aqueous solution in a 500 milliliter reaction flask, then add 13.5 grams of p-cymeniline, 10 milliliters of concentrated hydrochloric acid and 20.8 grams of hydroxylamine hydrochloride, heat the mixture to 60 ° C, After reacting for 3 hours, it was cooled to room temperature and filtered to obtain 4-isopropyloximinoacetoethylamine as a yellow-brown solid.
[0021] In a 250 ml reaction flask, add solid 4-isopropyloximinoacetoethylamine and 35 grams of concentrated sulfuric acid, stir to dissolve, heat to 80°C, react for 0.5 hours and cool to room temperature, add the reaction solution to ice water to precipitate, Filtration yielded 9.4 g of a reddish-brown 5-isopropyl-isatin solid.
[0022] In a 500ml reaction flask, add 10g of calcium oxide and 350ml of water, stir to dissolve, then add the ethanol solution of isatin obtained in the previous step, heat to 80°C, then add 9.3g of chloroacetone dropwise, and...
Embodiment 2
[0024] In a 500 ml reaction flask, add 33 g of chloral hydrate and 142 g of potassium sulfate in water, then add 13.5 g of p-cymeniline, 10 ml of concentrated hydrochloric acid and 34.7 g of hydroxylamine hydrochloride, and heat the mixture to 100°C. After reacting for 2 hours, it was cooled to room temperature and filtered to obtain 4-isopropyloximinoacetoethylamine as a yellow-brown solid.
[0025] In a 250 ml reaction flask, add solid 4-isopropyloximinoacetoethylamine and 70 g of concentrated sulfuric acid, stir to dissolve, stir and react at 60°C for 1 hour, cool down to room temperature, add the reaction solution to ice water to precipitate, Filtration yielded 9.4 g of a reddish-brown 5-isopropyl-isatin solid.
[0026] In a 500ml reaction flask, add 10g of calcium oxide and 350ml of water, stir to dissolve, add the ethanol solution of isatin obtained in the previous step, heat to 80°C, add 9.3g of chloroacetone dropwise, and continue the reaction at 100°C for 5 Hour. Th...
Embodiment 3
[0028] Add 49.5 grams of chloral hydrate and an aqueous solution of 142 grams of sodium sulfate in a 500 milliliter reaction flask, then add 13.5 grams of p-cymeniline, 10 milliliters of concentrated hydrochloric acid and 20.8 grams of hydroxylamine hydrochloride. The mixture was heated to 40° C., cooled to room temperature after 4 hours of reaction, and filtered to obtain 4-isopropyloximinoacetoethylamine as a yellow-brown solid. .
[0029] In a 250ml reaction bottle, add solid 4-isopropyloximinoacetoethylamine and 50 grams of concentrated sulfuric acid, stir to dissolve, heat to 70°C and stir for 0.5 hours, cool down to room temperature, add the reaction solution to ice water to precipitate , filtered to obtain 9.2 g of reddish-brown 5-isopropyl-isatin solid
[0030] In a 500 ml reaction bottle, add 10 grams of calcium hydroxide and 350 ml of water, stir to dissolve, add the ethanol solution of isatin obtained in the previous step, heat to 60 ° C, then add 13.9 grams of chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com