The synthetic method of 2-methyl-3-hydroxyquinoline and the preparation method of quinone ketone disperse dye
The technology of a kind of hydroxyquinoline and synthetic method is applied in the preparation of quinone disperse dyes and the synthesis field of 2-methyl-3-hydroxyquinoline, which can solve the problem of increasing the preparation cost of quinone disperse dyes and reducing the 2- Methyl-3-hydroxyquinoline yield, instability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
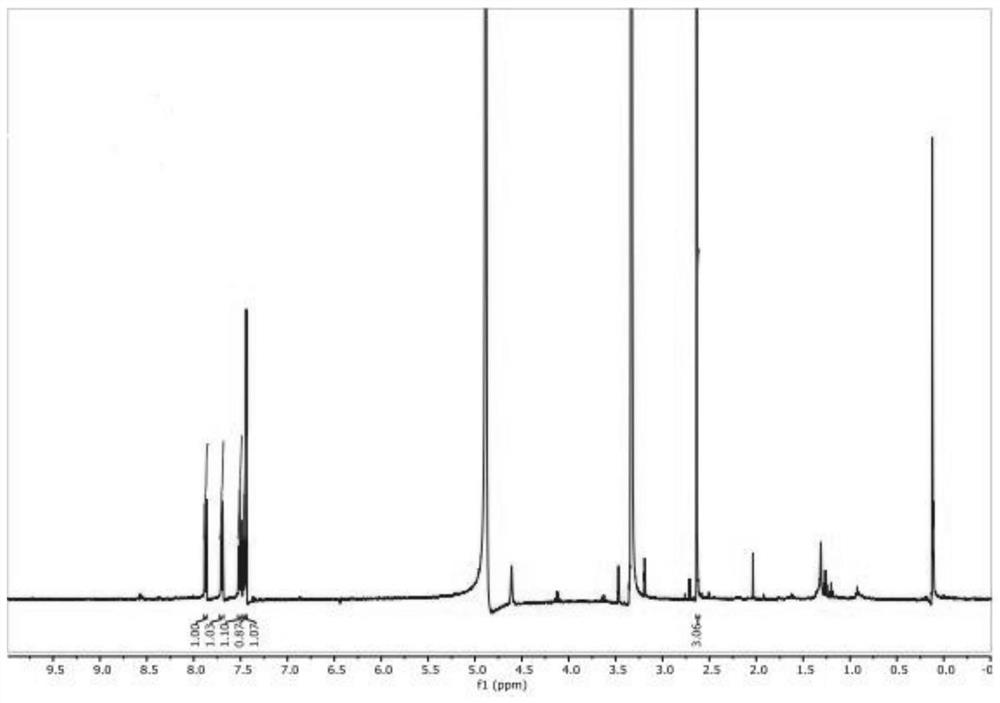
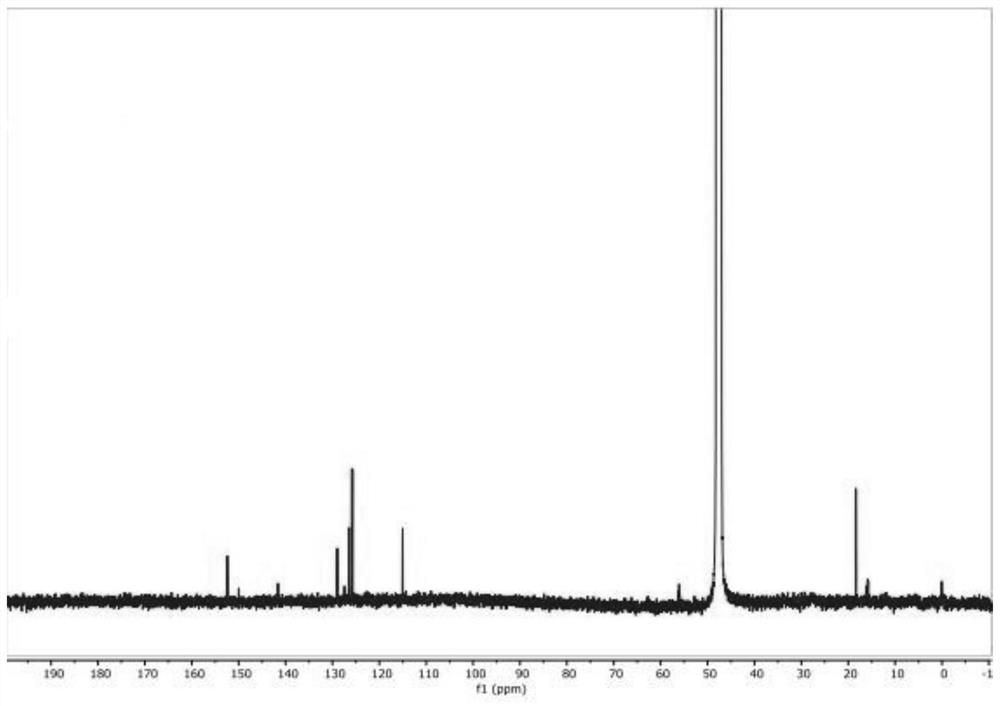
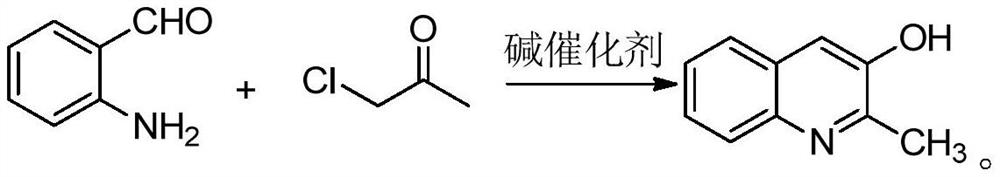
[0037] One embodiment of the present invention provides a synthesis method of 2-methyl-3-hydroxyquinoline, comprising the following steps S10-S20.
[0038] Step S10, mixing anthranilaldehyde, chloroacetone, base catalyst, phase transfer catalyst and solvent, and adjusting the pH value to 11-13 to obtain a mixed solution.
[0039] First, anthranilaldehyde, chloroacetone, alkali catalyst, phase transfer catalyst and solvent are mixed, and under the action of the phase transfer catalyst, all raw materials are fully contacted to obtain a homogeneously mixed solution, thereby improving the efficiency of subsequent reactions.
[0040] The technicians of the present invention have found through in-depth research: although o-aminobenzaldehyde and chloroacetone pass through the microchannel reaction under the effect of an alkali catalyst, the ring-forming reaction can occur rapidly to obtain 2-methyl-3-hydroxyquinoline; yet, During the microchannel reaction process, the reactivity of o...
Embodiment 1
[0089] 1) Anthranilaldehyde, chloroacetone and tetrabutylammonium bromide are dissolved in tetrahydrofuran to form a first solution; sodium hydroxide and zinc oxide are dissolved in water to form a second solution. The first solution and the second solution are then mixed to obtain a mixed solution. Wherein, the mass ratio of o-aminobenzaldehyde, chloroacetone, tetrabutylammonium bromide, sodium hydroxide and zinc oxide in the mixed solution is: 1:0.6:0.1:0.6:0.08. The pH value of the mixed solution = 12.1.
[0090] 2) Pump the mixed solution obtained in step 1) into a preheater to preheat to 30°C to obtain a preheated reaction solution; then pump the preheated reaction solution into a microchannel reactor, and keep warm at 30°C for reaction, wherein The flow rate of the mixed solution is 50mL / min; the product obtained by the reaction enters the separation tank for separation treatment to obtain 2-methyl-3-hydroxy-quinoline.
[0091] 3) Calculate the yield of 2-methyl-3-hydr...
Embodiment 2
[0095] 1) Dissolving o-aminobenzaldehyde, chloroacetone and tetrabutylammonium bromide in tetrahydrofuran to form a first solution; dissolving sodium hydroxide and zinc oxide in water to form a second solution. The first solution and the second solution are then mixed to obtain a mixed solution. Wherein, the mass ratio of o-aminobenzaldehyde, chloroacetone, tetrabutylammonium bromide, sodium hydroxide and zinc oxide in the mixed solution is: 1:0.6:0.2:0.9:0.08. The pH value of the mixed solution = 12.8.
[0096] 2) Pump the mixed solution obtained in step 1) into a preheater to preheat to 30°C to obtain a preheated reaction solution; then pump the preheated reaction solution into a microchannel reactor, and keep warm at 30°C for reaction, wherein The flow rate of the mixed solution is 50mL / min; the product obtained by the reaction enters the separation tank for separation treatment to obtain 2-methyl-3-hydroxy-quinoline.
[0097] 3) Calculate the yield of 2-methyl-3-hydroxyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com