1,1,1-trichloroacetone preparation method
A technology of trichloroacetone and chloroacetone, which is applied in the field 1, can solve problems such as difficult operability, and achieve the effects of convenient operation, convenient raw materials, and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
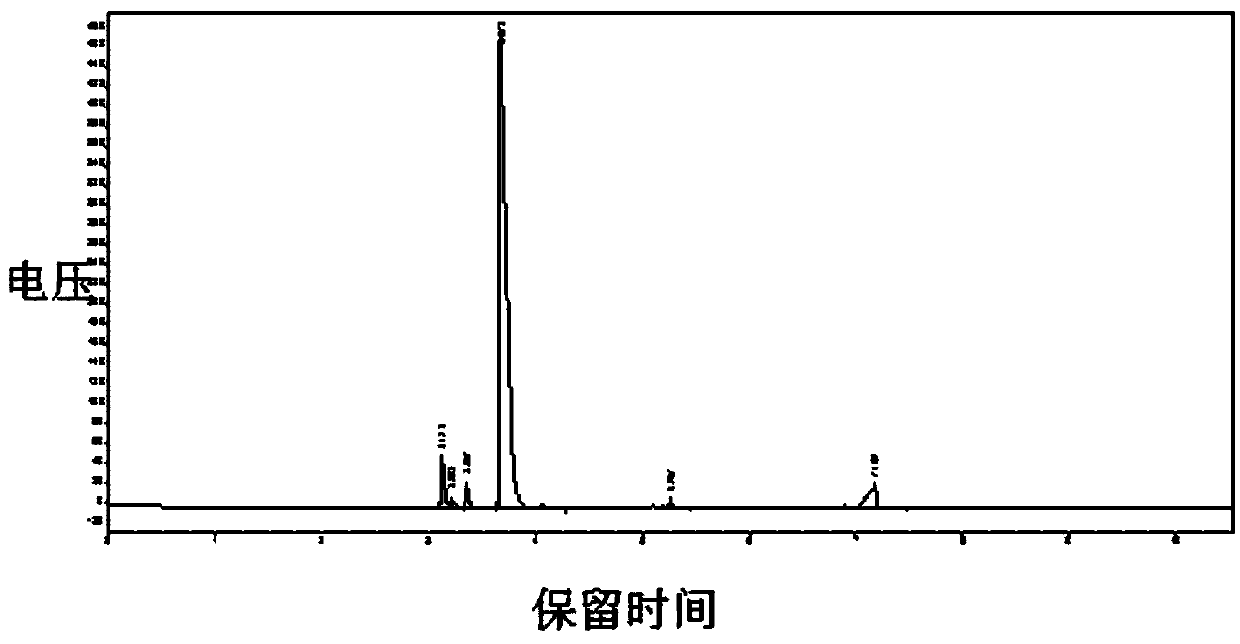
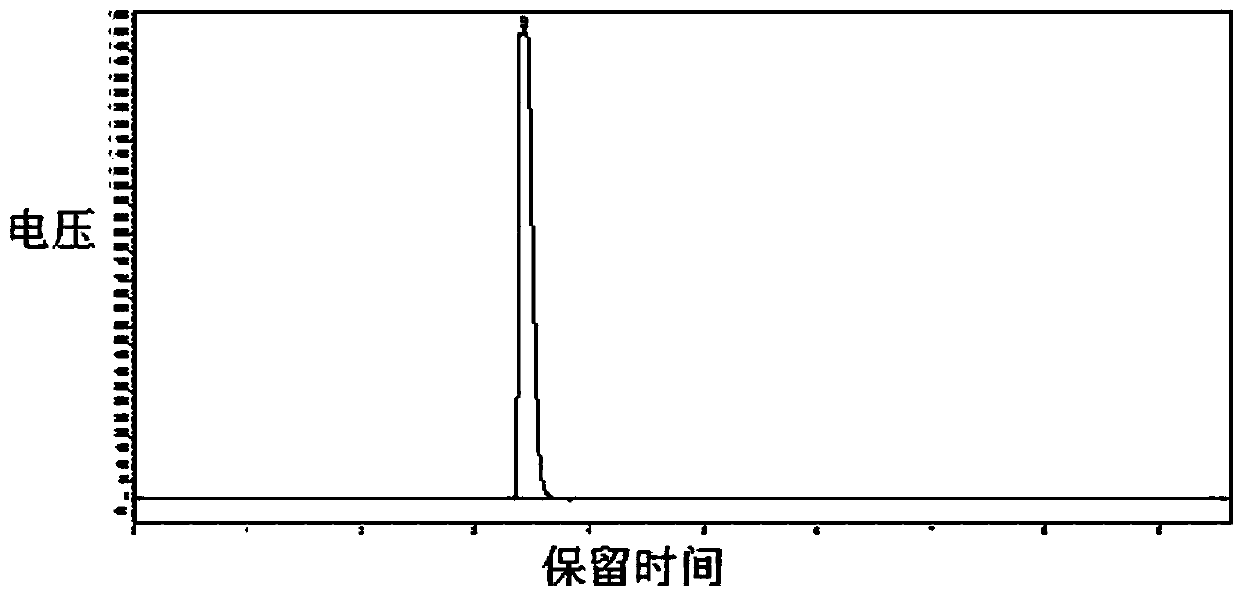
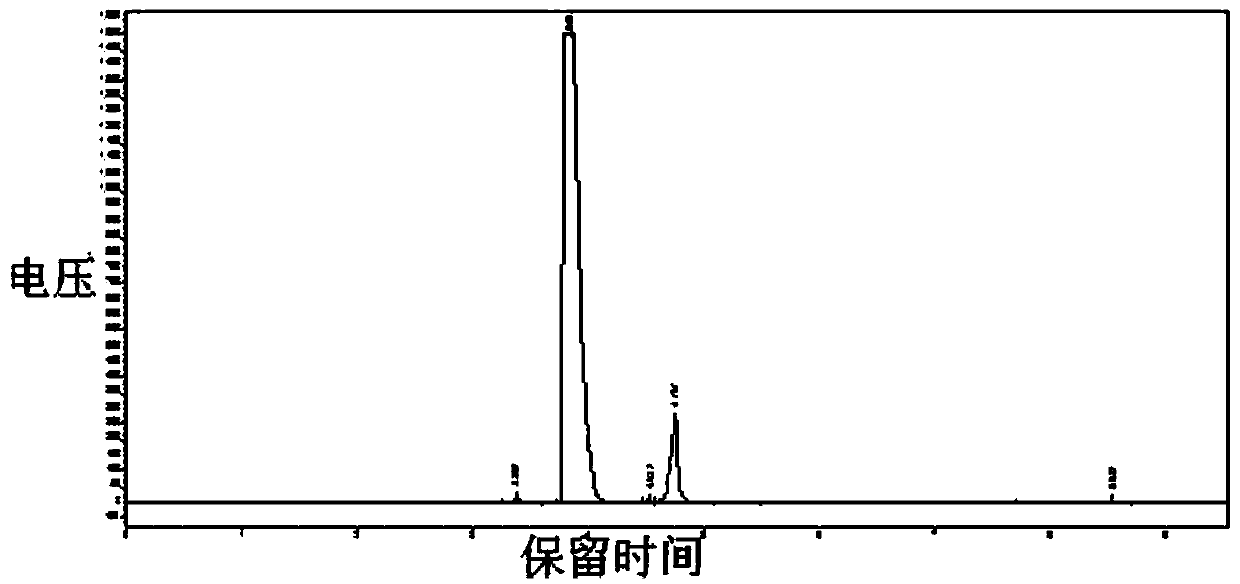
Image
Examples
preparation example Construction
[0026] The preparation method of 1,1,1-trichloroacetone of the present invention comprises the following steps:
[0027] Step 1, use a large amount of aqueous solution, drop into monochloroacetone and water, do not use other organic solvents; Aqueous solution and raw material monochloroacetone form a kind of water-oil two-phase system or water-monochloroacetone-undissolved basic compound three-phase system;
[0028] Step 2, the basic compound forms an aqueous solution or suspension in water;
[0029] Step 3, feed chlorine gas under stirring until the solution is clear;
[0030] Step 4, during the whole reaction process, the temperature is controlled at 0 to 25 degrees Celsius;
[0031] Step 5, after the reaction is over, let it stand still, separate the lower oil phase, wash with water, then dry with anhydrous calcium chloride or anhydrous sodium sulfate or anhydrous magnesium sulfate, filter off the desiccant, and obtain products 1, 1, 1 - Trichloroacetone.
[0032] The b...
Embodiment 1
[0038] In a 5-liter reaction kettle with mechanical stirring (externally connected to an exhaust gas absorption device), 231 grams of monochloroacetone, 564 grams of sodium bicarbonate, and 1080 grams of water were successively put in, the temperature was controlled at 20 to 24 degrees Celsius, and chlorine gas was passed for 6 hours under stirring. (About 400 grams according to the flowmeter calculation), the solution system becomes clear, put it aside for 1 hour, and separate the liquids, and the yellow-green organic phase liquid in the lower layer of the gained is dried with 200 grams of anhydrous calcium chloride to obtain the product 1,1,1-trichloro 298 grams of acetone, yield 74%; gas phase purity 85%, moisture<0.1%.
Embodiment 2
[0040] In a 5-liter reaction kettle with mechanical stirring (externally connected to an exhaust gas absorption device), 231 grams of monochloroacetone, 564 grams of sodium bicarbonate, and 1080 grams of water are successively dropped into, and the temperature is controlled at 10 to 20 degrees Celsius, and the chlorine gas is passed for about 7 hours under stirring. Hour (calculate about 400 grams according to flow meter), solution system becomes clarified, leaves standstill 1 hour, liquid separation, the yellow-green organic phase liquid of gained lower floor is dried with 200 grams of calcium chloride anhydrous, obtains product 1,1,1-three Chloroacetone 297 grams; gas phase purity 85%, water <0.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com