Preparation method of tralopyril
A molar ratio and equation technology, applied in the agricultural field, can solve the problems of low safety factor, many impurities, low yield, etc., and achieve the effect of less impurities, high purity and high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
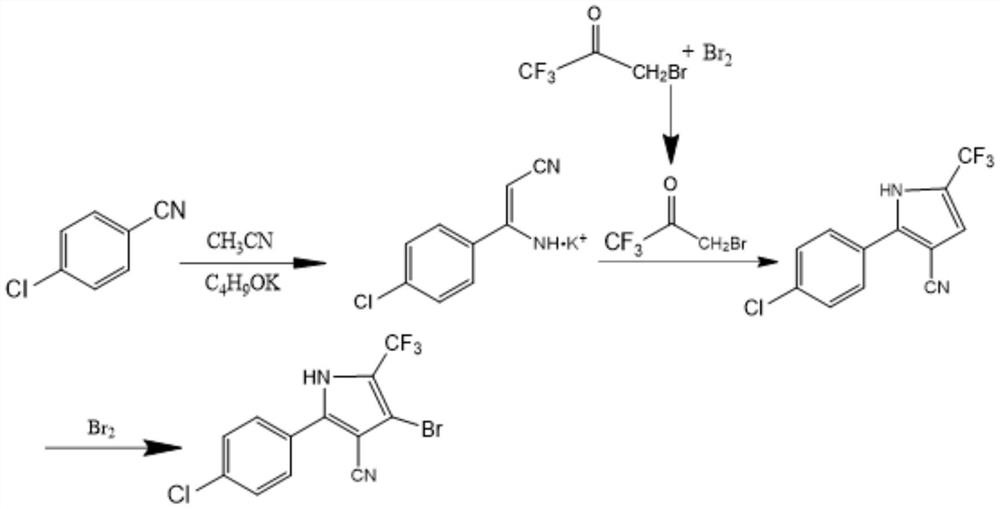
[0028] The specific reaction process of the salt-forming reaction is described in detail in this example, and the alkali metal salt of p-chlorophenylaminoacrylonitrile can be successfully synthesized according to this synthesis method.
[0029] Put 112g of 1,1,1-trifluoroacetone (molecular weight 112.05) and 560mL of dichloroethane into a transparent reactor, transfer the reactor to a custom-made microwave oven and heat it to 60°C, and at the same time turn on a 300w quartz lamp or mercury lamp to irradiate the transparent Reactor, add bromine dropwise into the transparent reactor at a molar ratio of 1:1.06, under this condition, keep it for 5 hours, take a sample and control it, when the remaining 1,1,1-trifluoroacetone is ≤0.5%, the control is qualified . After the central control is qualified, add 168g of water, stir and wash for 0.5h, let stand to separate layers, separate the water layer, add 112mL dichloroethane to the water layer for extraction, combine the extract with...
Embodiment 2
[0031] The specific reaction process of the one-step bromination reaction is described in detail in this example, and 1-bromo-3,3,3-trifluoroacetone can be successfully synthesized according to this synthesis method.
[0032] Put 137.6g of p-chlorobenzonitrile crystals and 350mL of acetonitrile into the reactor, add potassium tert-butoxide at a molar ratio of 1:1.03, stir and raise the temperature to 70°C, and keep it warm for 7h. Filter out the resulting potassium salt through a funnel, and the filter cake is the potassium salt of p-chlorophenylaminoacrylonitrile, which is dried for later use.
[0033] The filtrate is a mixture of acetonitrile and by-product tert-butanol. After rectification, acetonitrile can be recycled.
Embodiment 3
[0035] The specific reaction process of the ring-forming reaction is described in detail in this embodiment. According to this synthetic method, 2-(4-chlorophenyl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile (pyrrole nitrile).
[0036] Add 216.7g of p-chlorophenylaminoacrylonitrile potassium salt, 650mL of cyclohexane and 5g of p-toluenesulfonic acid into the reactor, raise the temperature to 55°C, and add 1-bromo-3 , 3,3-Trifluoroacetone, after the dropwise addition is completed, keep warm at this temperature for 4 hours, take a sample for central control, and the remaining potassium salt ≤ 0.5% of the central control is qualified. After the central control is qualified, 250mL of water is added to the reactor, washed twice, and then separated into layers. The water layer is wastewater containing potassium bromide salt. The organic layer was distilled under normal pressure and reduced pressure to obtain a crude product, which was recrystallized by adding 350 mL of methanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com