Acryloyl group-containing nuclear transport modulator and uses thereof
一种烷基、基团的技术,应用在CRM1蛋白调节剂领域,能够解决代谢性质差、毒副作用强、易穿过等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
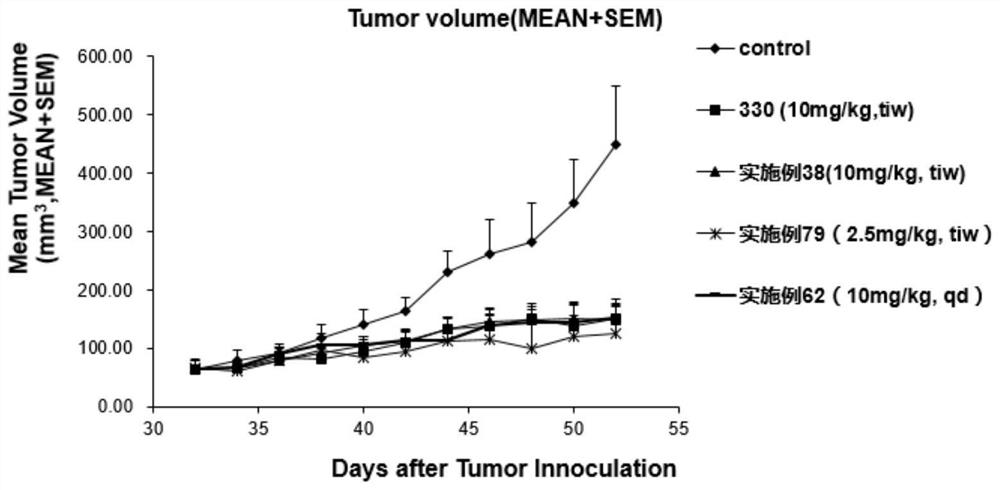
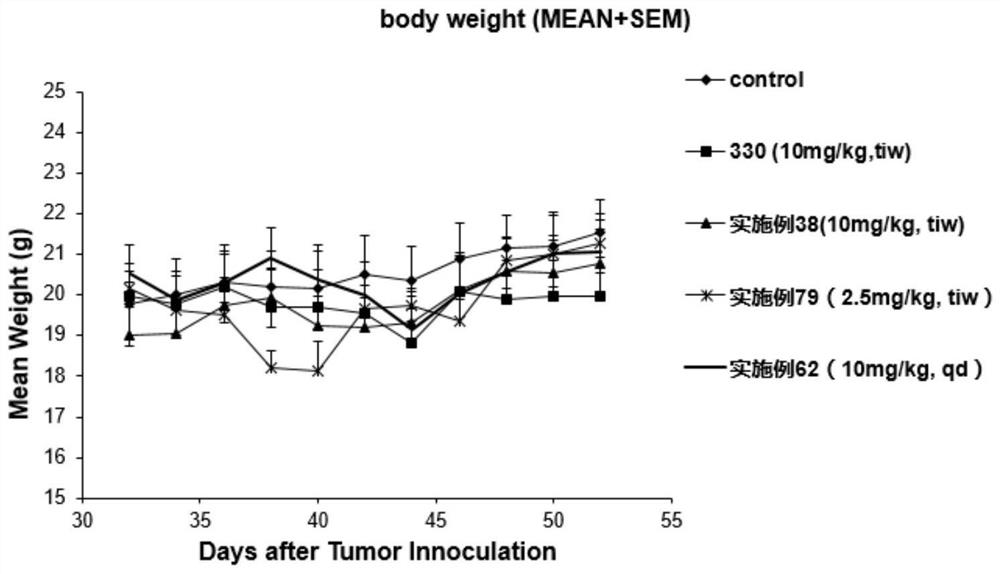
Image
Examples
preparation example 1
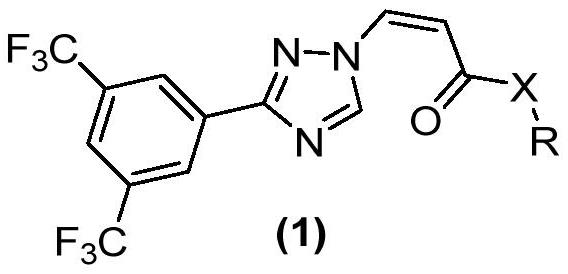
[0153] Production Example 1 (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)acrylic acid (I-1) synthesis
[0154]
[0155] Synthesis of 3,5-bis(trifluoromethyl)thiobenzamide (M2)
[0156] 3,5-Bis(trifluoromethyl)benzonitrile (47.82g, 0.2mol) was dissolved in DMF (250mL), NaSH (22.42g, 2.0eq) and MgCl were added 2 (38.08g, 2.0eq), stirred at room temperature for 3h, the mixture was poured into ice-water (2L), extracted with EA (250mL*3), the organic layers were combined, washed with saturated aqueous sodium chloride solution (100mL*2), after Dry over sodium sulfate, filter and concentrate under reduced pressure to obtain the crude product 3,5-bis(trifluoromethyl)thiobenzamide (46.97g, yield 86%).
[0157] Synthesis of 3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazole(M3)
[0158] 3,5-bis(trifluoromethyl)thiobenzamide (46.44g, 0.17mol) was dissolved in DMF (250mL), and hydrazine hydrate (16.5mL, 2.0eq) was added dropwise, and stirring was continued for 1...
Embodiment 1
[0164]Example 1 (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N-(2-oxo Synthesis of azetidinyl-1-yl)acrylamide (compound 1)
[0165]
[0166] (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)acrylic acid (105mg, 0.3mmol) and 1- Aminoazetidinyl-2-one (39mg, 0.45mmol) was dissolved in dry EA (10mL), under nitrogen protection and cooled to -60°C, the one in the reaction system was added to T 3 P (0.3mL, 2M EA solution), then add DIPEA (77mg, 0.6mmol), and continue to stir the reaction for 3h, add a little ice water to quench the reaction, wash with water (20mL), and extract the aqueous phase with EA (20mL*2) , combined organic layers, washed with saturated sodium chloride solution (20mL), dried over anhydrous sodium sulfate, concentrated under reduced pressure and purified by column chromatography (DCM / MeOH=1 / 100 to 1 / 30) to obtain product (Z)-3 -(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N-(2-oxoazetidinyl-1 -yl)acrylamide ...
Embodiment 2-89
[0168] Synthesis of Embodiment 2-89 (Compound 2-89)
[0169] Using I-1 as raw material, reacting with different hydrazides, similar to the synthesis of compound 1, compound 2-89 can be obtained.
[0170] Table 2. Mass spectrum and NMR data of compound 2-71
[0171]
[0172]
[0173]
[0174]
[0175]
[0176]
[0177]
[0178]
[0179]
[0180]
[0181]
[0182]
[0183]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com