Au/M1-M2-Ox/Al2O3 nano-gold catalyst for catalyzing oxidation of CO in CO2-rich atmosphere
A catalyst, nano-gold technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, catalyst activation/preparation, etc., can solve the problem of reducing the activity and stability of gold catalyst, carbonate species Accumulation, decreased reactivity and other problems, to achieve the effect of improving catalytic activity and reaction stability, reducing strong basic sites, and improving catalytic reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1%Au / 8wt.% / Fe-Ce-O x / Al 2 o 3 Preparation of:
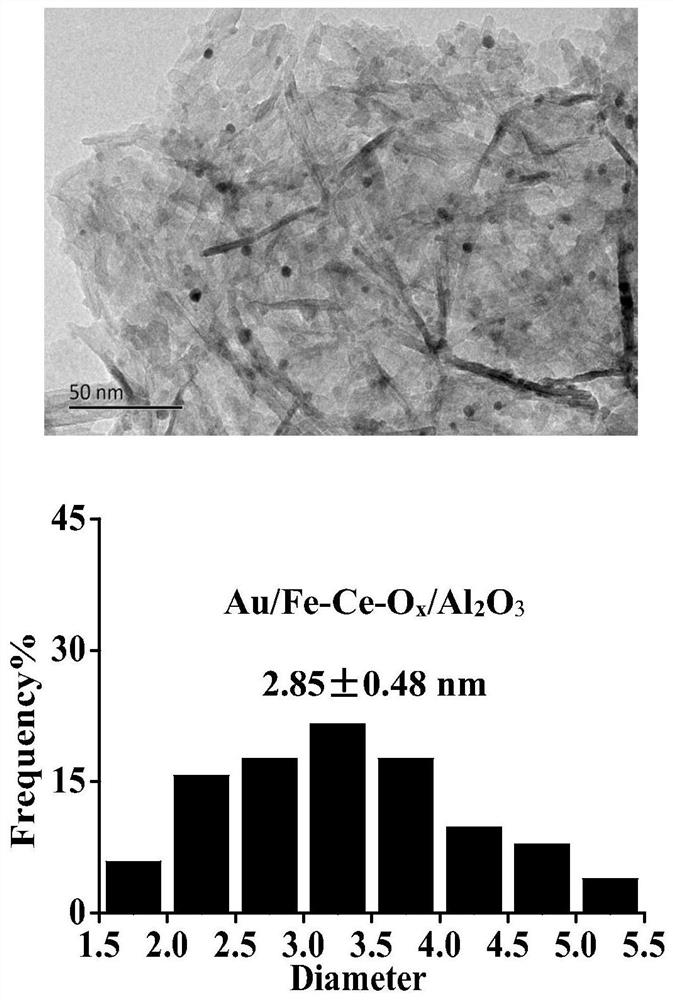
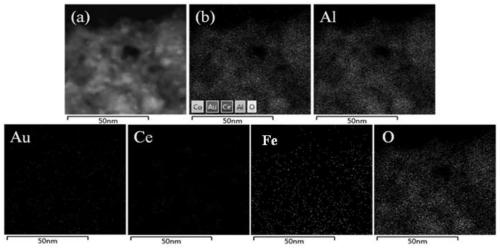
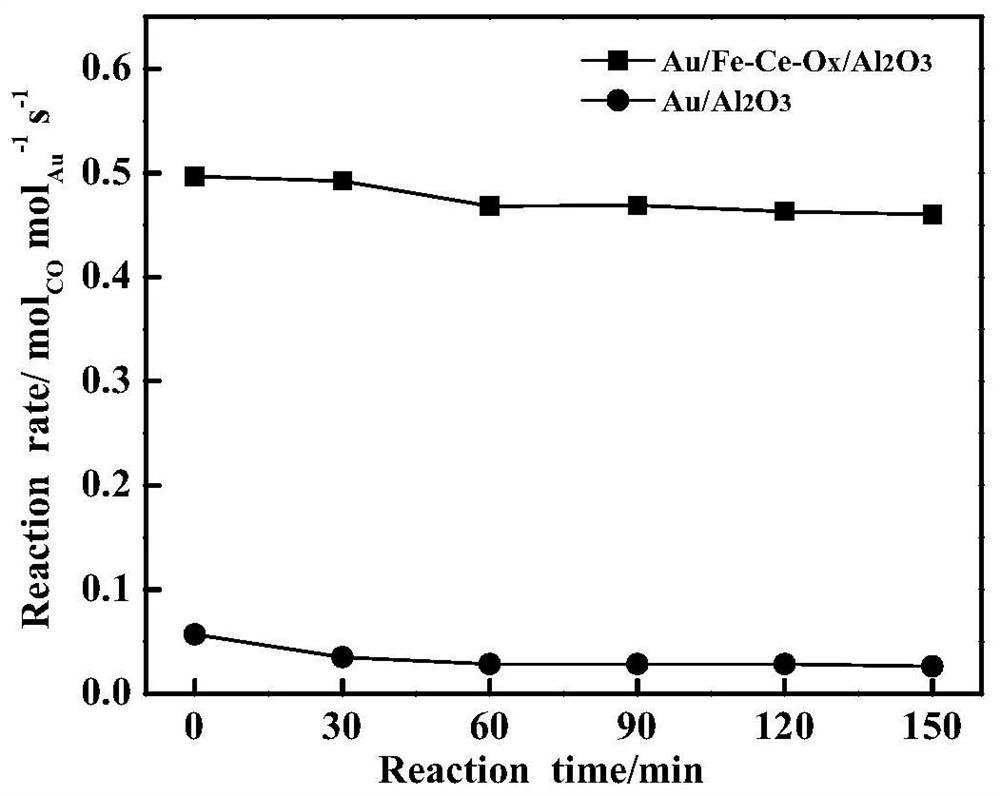
[0029] 0.20ml of Ce(NO 3 ) 2 solution (0.601mol / L) and 0.19 ml of Fe(NO 3 ) 3 The solution was mixed and diluted to 1.5 ml with deionized water; 0.92 g γ-Al 2 o 3 Mix well with the solution and age for several hours; dry the sample at 120°C for 2h, then dry it at 650 o Calcined at C for 4 hours, the obtained composite support is Fe-Ce-O x / Al 2 o 3 , into powder. 0.516 gHAuCl 4 Solution (19.12 g Au / L) is placed in a beaker, and its pH value is adjusted to 9 by adding ammonia water dropwise; the Fe-Ce-O x / Al 2 o 3 The carrier was added to the solution and stirred for several hours; the sample was rinsed with deionized water to remove residual chloride ions, and dried in air at 70 °C for several hours, and the obtained sample was Au / Fe-Ce-O x / Al 2 o 3 . Among them, the TEM photos and the particle size distribution of Au nanoparticles are as follows figure 1 as shown, figure 1 The Au nanoparticles sh...
Embodiment 2
[0041] 1%Au / 7wt.% / Fe-Mn-O x / Al 2 o 3 Preparation of:
[0042] Weigh 0.93 g Al 2 o 3 Dissolve the powder in 30 mL of ionized water and mix well, add 0.50 mL of Fe(NO 3 ) 3 solution (0.612 mol / L) and 0.27 mL Mn(NO 3 ) 2 solution (1.4059 mol / L) and fully mixed; filter the suspension under reduced pressure, and place the filter cake in an oven for 120 o C drying for 2 h, and at 650 oC Calcined for 4 h and ground to obtain Fe-Mn-O x / Al 2 o 3 Composite carrier. 0.516 g HAuCl 4 Solution (19.12 g Au / L.) is placed in a beaker, and its pH value is adjusted to 9 by adding ammonia water dropwise; the Fe-Mn-O x / Al 2 o 3 The carrier was added to the solution and stirred for several hours; the sample was rinsed with deionized water to remove residual chloride ions, and dried in air at 70 °C for several hours, and the obtained sample was expressed as Au / Fe-Mn-O x / Al 2 o 3 . After the sample was reacted for 1000 minutes, the raw gas composition was: 60 vol.% CO 2 +1...
Embodiment 3
[0044] 1%Au / 5wt.% / Ni-Nb-O x / Al 2 o 3 Preparation of:
[0045] 0.44ml of Ni(NO 3 ) 2 solution (0.701mol / L) and 0.19 ml of Nb(NO 3 ) 3 solution (1.05mol / L) was mixed and diluted to 1.5ml with deionized water; 0.95g γ-Al 2 o 3 Mix well with the solution and age for several hours; dry the sample at 100°C for 4 hours, then dry it at 650 o Calcined at C for 4 hours, the obtained composite support is Ni-Nb-O x / Al 2 o 3 , into powder. 0.516 g HAuCl 4 Solution (19.12 g Au / L.) is placed in a beaker, and the pH value is adjusted to 10 by adding ammonia water dropwise; the Ni-Nb-O x / Al 2 o 3 The carrier was added to the solution and stirred for several hours; the sample was rinsed with deionized water to remove residual chloride ions, and dried overnight in air at 60 °C, and the obtained sample was expressed as Au / Ni-Nb-O x / Al 2 o 3 . After the sample reacted for 1200 minutes, the raw gas composition was: 60 vol.% CO 2 +1 vol.% CO+0.5 vol.% O 2 , balanced N 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com