Kit for detecting foreign plasmid residues based on resistance gene and use method of kit
A resistance gene and kit technology, applied in the field of detection kits for exogenous plasmid residues, can solve the problems of limited infection efficiency, tumorigenic risk, long cycle, etc., and achieve good repeatability, good specificity, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Screening of primer pairs for specific amplification of Kana resistance gene
[0097] 1. Design multiple sets of primer pairs based on Kana resistance gene and commission Shanghai Jereh Biotechnology Co., Ltd. to synthesize. The sequence of the primer pair is shown in Table 1 below.
[0098] Table 1: Sequences of primer pairs 1-4
[0099]
[0100]
[0101] The sequence of the Kana resistance gene is shown in SEQ ID NO:9.
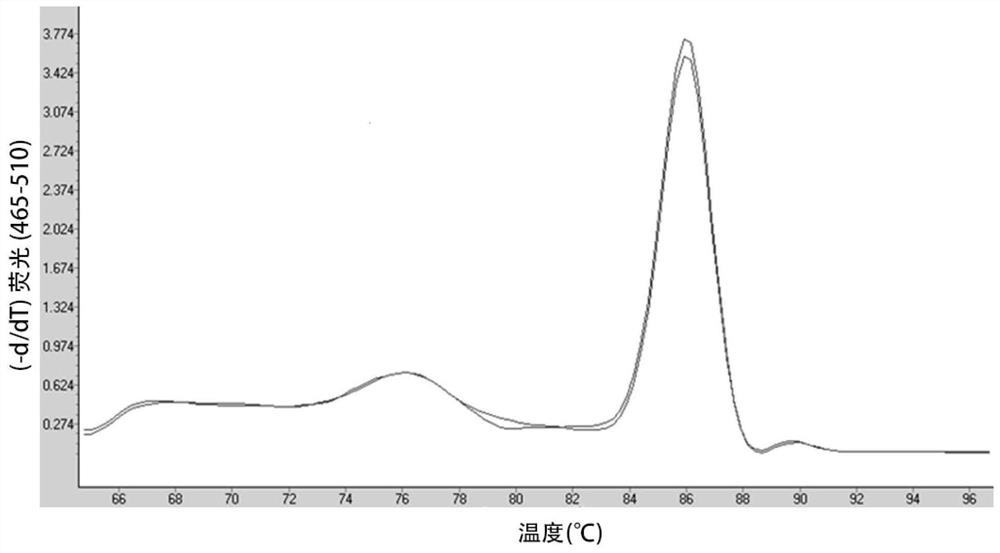
[0102] 2. The specificity and sensitivity screening of primers
[0103] Samples, reagents, instruments
[0104] Instrument: LightCycle480 fluorescent quantitative PCR detector (Roche)
[0105] Sample: Shanghai Jierui Biotechnology Co., Ltd. was commissioned to synthesize CD19CAR exogenous gene (including CD8 signal peptide, scFv, CD8 transmembrane region, CD8 hinge region, 4-1BB and CD3B). The sequence is shown in SEQ ID NO: 14 and is included in it. Introduce a polyclonal restriction site (BglII-XbaI-EcoRI-BamHI) upstream, insert a restriction site ...
Embodiment 2
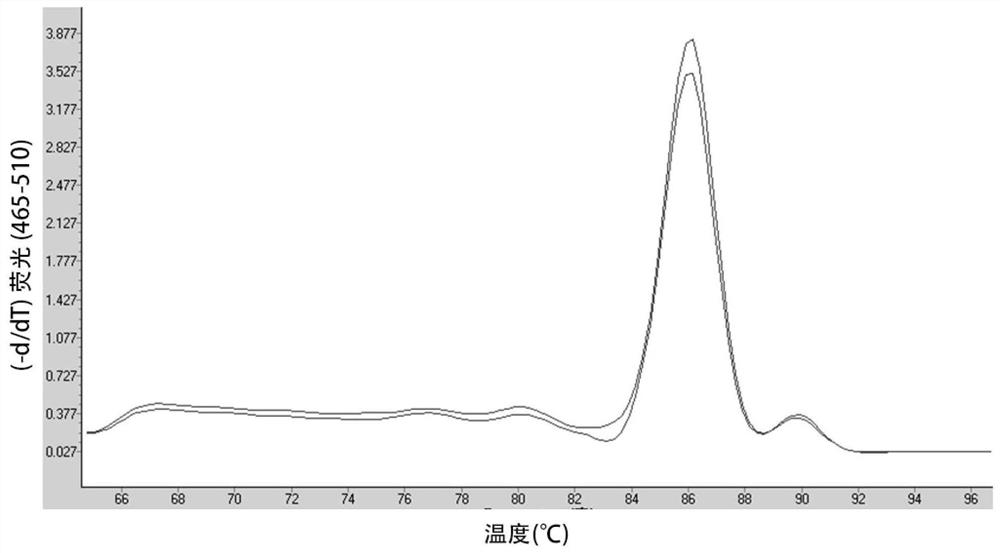
[0125] Example 2 According to the preferred primer pairs, design probes to detect amplification efficiency
[0126] 1. Design the probes for the amplification of Kana resistance gene and the amplification primers and probes for the internal reference Actin gene. The sequence is shown in Table 5, and the synthesis is commissioned by Shanghai Jereh Biotechnology Co., Ltd. The nucleotides with capital letters in the probe A sequence are locked nucleic acid modified nucleotides.
[0127] Table 5: Primers and probe sequences
[0128]
[0129] Samples, reagents, instruments
[0130] Instrument: LightCycle480 fluorescent quantitative PCR detector (Roche)
[0131] Sample: Plasmid pNB328-CD19CAR (CD19CAR)
[0132] Reagents: Genome extraction kit (Takara), Taqman Master Mix reagent (ABI).
[0133] Take the aforementioned plasmid pNB328-CD19CAR (CD19CAR) as a template, the preferred primer pair 4 and probe A combination; configure the reaction solution according to the reaction system of the fluore...
Embodiment 3
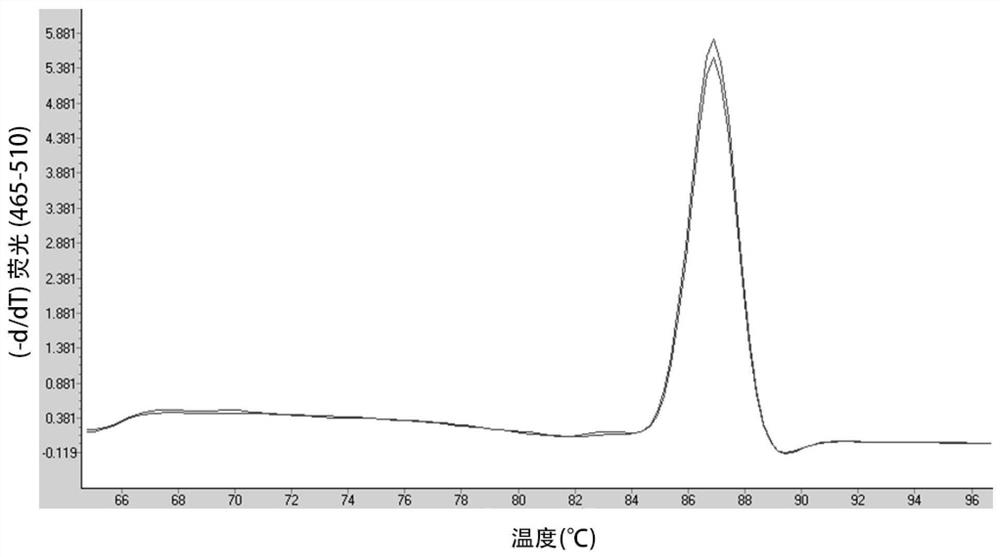
[0141] Example 3: Detection of the residual amount of resistance genes in CAR-T cells
[0142] Instrument: LightCycle480 fluorescent quantitative PCR detector (Roche),
[0143] Samples: 4 CD19CAR-T samples. CD19CAR-T cells were constructed as follows: Peripheral blood mononuclear cells (PBMCs) were isolated and obtained by conventional methods in the field. The PBMCs were adhered and cultured for 2-4 hours, among which the non-adherent suspended cells were the initial T cells. The suspended cells were collected in a 15ml centrifuge tube, centrifuged at 1200rpm for 3min, discarded the supernatant, added physiological saline, centrifuged at 1200rpm for 3min, discarded the physiological Saline, and repeat this step; take a 1.5ml centrifuge tube, add 5 heart tubes, 6 Cells were centrifuged at 1200rpm for 3min, the supernatant was discarded, and the electroporation kit (purchased from Lonza) was added. A total of 100μl of electroporation reagent and 4μg of pNB328-CD19CAR plasmid were ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com