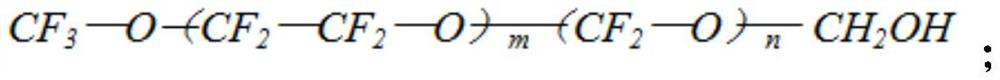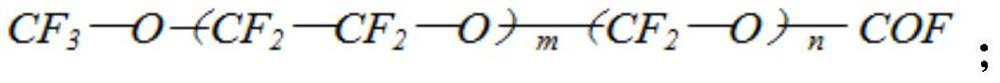Straight-chain polyperfluoroether compound with hydroxyl-containing terminal group and preparation method thereof
A polyperfluoroether and compound technology, which is applied in the field of linear perfluoropolyether compounds, can solve problems such as affecting the integrity of molecular arrangement, and achieve the effect of neat molecular structure, smooth compound, and good adaptability to high and low temperature changes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Preparation of linear perfluoropolyetheryl fluoride
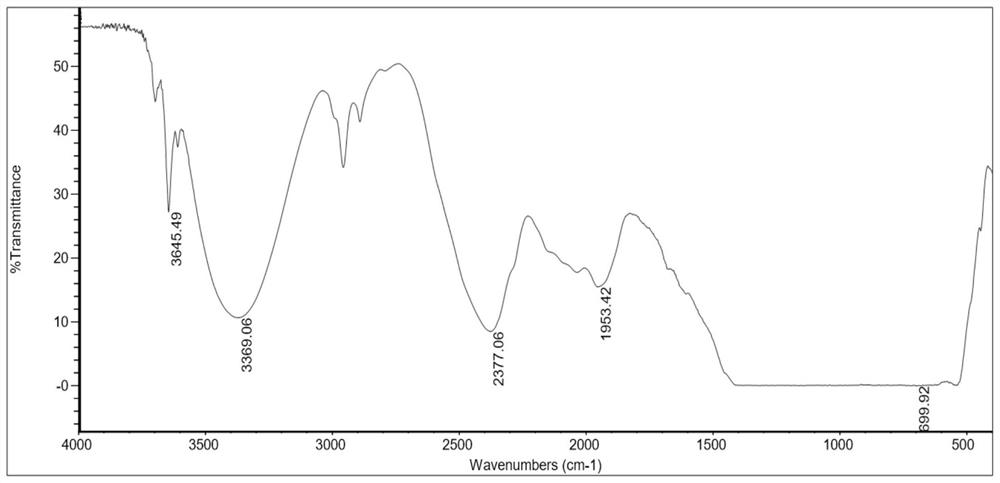
[0035] Add tetrafluoroethylene gas to the reactor, and feed oxygen into the reactor at the same time, control the volume ratio of tetrafluoroethylene and oxygen to 1:4, control the temperature of the reactor at -50°C, and carry out photooxidative polymerization under ultraviolet light Reaction, the reaction time is 28 hours, and the linear perfluoropolyether product whose terminal group is acid fluoride is obtained, the structural formula is shown in formula II, wherein m is 59, and n is 100.
[0036] 2) Esterification reaction of linear perfluoropolyetheryl fluoride
[0037] Taking triethylamine as the acid-binding agent, the volume ratio of triethylamine and the end group obtained in step 1) is a linear perfluoropolyether of acid fluoride is 2:1, and the product obtained in step 1) and ethanol are according to 1:1 The molar ratio of 4 was reacted at 60°C for 7 hours. After cooling to room temperature, the obtai...
Embodiment 2
[0041] 1) Preparation of linear perfluoropolyetheryl fluoride
[0042] Add tetrafluoroethylene gas to the reactor, and feed oxygen into the reactor at the same time, control the volume ratio of tetrafluoroethylene and oxygen to 1:3, control the temperature of the reactor at -40°C, and carry out photooxidative polymerization under ultraviolet light Reaction, the reaction time is 35 hours, and the linear perfluoropolyether product whose terminal group is acid fluoride is obtained, the structural formula is shown in formula II, wherein m is 130, and n is 62.
[0043] 2) Esterification reaction of linear perfluoropolyetheryl fluoride
[0044] Taking triethylamine as the acid-binding agent, the volume ratio of triethylamine and the end group obtained in step 1) is a linear perfluoropolyether of acid fluoride is 2:1, and the product obtained in step 1) and ethanol are according to 1:1 The molar ratio of 4 was reacted at 50°C for 11 hours. After cooling to room temperature, the obta...
Embodiment 3
[0048] 1) Preparation of linear perfluoropolyetheryl fluoride
[0049] Add tetrafluoroethylene gas to the reactor, and feed oxygen into the reactor at the same time, control the volume ratio of tetrafluoroethylene and oxygen to 1:6, control the temperature of the reactor at -70°C, and carry out photooxidative polymerization under ultraviolet light Reaction, the reaction time is 35 hours, and the linear perfluoropolyether product whose terminal group is acid fluoride is obtained, the structural formula is shown in formula II, wherein m is 200, and n is 38.
[0050]2) Esterification reaction of linear perfluoropolyetheryl fluoride
[0051] Taking triethylamine as the acid-binding agent, the volume ratio of triethylamine and the end group obtained in step 1) is a linear perfluoropolyether of acid fluoride is 4:1, and the product obtained in step 1) is mixed with methanol according to 1:1 The molar ratio of 3 was reacted at 70°C for 7 hours. After cooling to room temperature, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com