Lead storage battery formation method
A chemical formation method and technology for lead-acid batteries, applied in the direction of lead-acid batteries, lead-acid battery construction, secondary batteries, etc., can solve the problems of short battery life and low α-PbO2 content, and achieve extended life, rapid formation, and increased content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
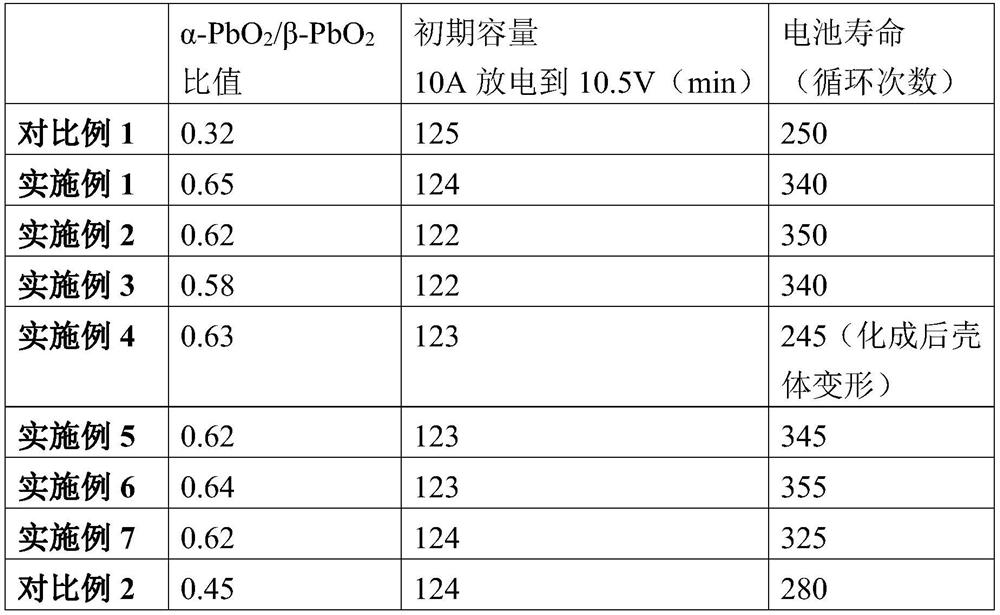
Examples
Embodiment 1
[0022] 1. Preparation of sodium sulfate aqueous solution
[0023] Prepare an aqueous solution with a mass concentration of sodium sulfate of 0.4%.
[0024] 2. Initial formation
[0025] Prepare the 20Ah battery with the assembled raw board, add the prepared aqueous solution containing sodium sulfate to the battery, add 1.2 times the volume of saturated liquid absorption, put the battery in a vacuum box after the addition, and connect the power cord to the battery Formation, the vacuum degree is controlled at -0.08MPa (the reading on the vacuum pressure gauge refers to the pressure smaller than the atmospheric pressure when the atmospheric pressure is taken as the reference, and the negative value indicates the vacuum degree), the formation current is controlled at 1.5C, and the formation time is 2h, so , the formation temperature can be precisely controlled at 60°C, and it is easy to form α-PbO under the initial alkaline condition 2 .
[0026] 3. The second step of formatio...
Embodiment 2
[0031] 1. Preparation of sodium sulfate aqueous solution
[0032] Prepare an aqueous solution with a mass concentration of sodium sulfate of 0.4%.
[0033] 2. Initial formation
[0034] Prepare the 20Ah battery with the assembled raw board, add the prepared aqueous solution containing sodium sulfate to the battery, the volume added is 1.4 times the saturated liquid absorption capacity, after adding, put the battery in a vacuum box, and connect the power cord Carry out battery formation, the vacuum degree is controlled at -0.08MPa (the reading on the vacuum pressure gauge refers to the pressure less than the atmospheric pressure when the atmospheric pressure is taken as the reference, and the negative value indicates the vacuum degree), the formation current is controlled at 1.0C, and the formation time is 3h , so that the formation temperature can be precisely controlled at 60°C, and it is easy to form α-PbO under the initial alkaline condition. 2 .
[0035] 3. The second s...
Embodiment 3
[0040] 1. Preparation of sodium sulfate aqueous solution
[0041] Prepare an aqueous solution with a mass concentration of sodium sulfate of 0.4%.
[0042] 2. Initial formation
[0043] Prepare the 20Ah battery with the assembled raw board, add the prepared aqueous solution containing sodium sulfate to the battery, the volume added is 1.4 times the saturated liquid absorption capacity, after adding, put the battery in a vacuum box, and connect the power cord For battery formation, the vacuum degree is controlled at -0.089MPa (the reading on the vacuum pressure gauge refers to the pressure less than the atmospheric pressure when the atmospheric pressure is taken as the reference, and the negative value indicates the vacuum degree), the formation current is controlled at 1.5C, and the formation time is 2h , so that the formation temperature can be precisely controlled at 50°C, and it is easy to form α-PbO under the initial alkaline condition. 2 .
[0044] 3. The second step o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com