Stable crystal form of avibactam sodium and preparation method thereof
A crystal form and stable technology, applied in the field of drug-stabilized crystal form of Avibatana and its preparation, can solve problems such as being unsuitable for industrial production, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
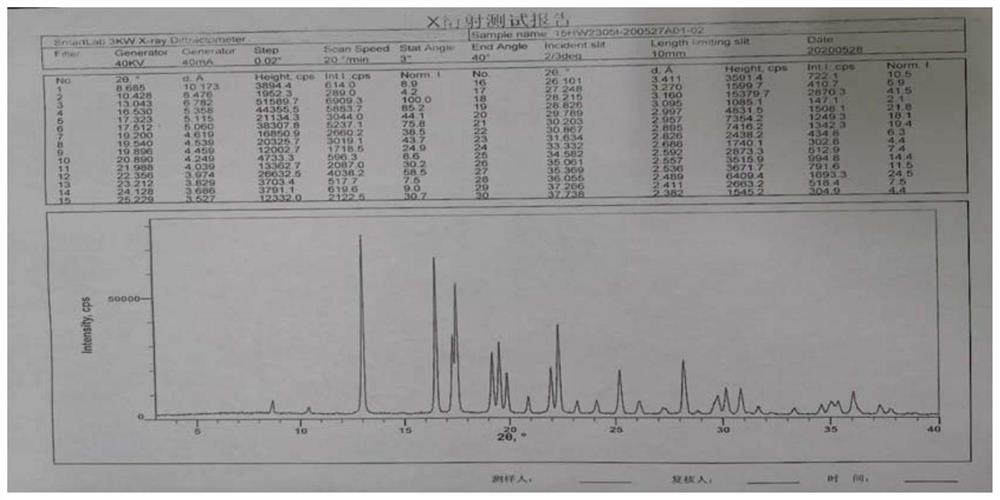
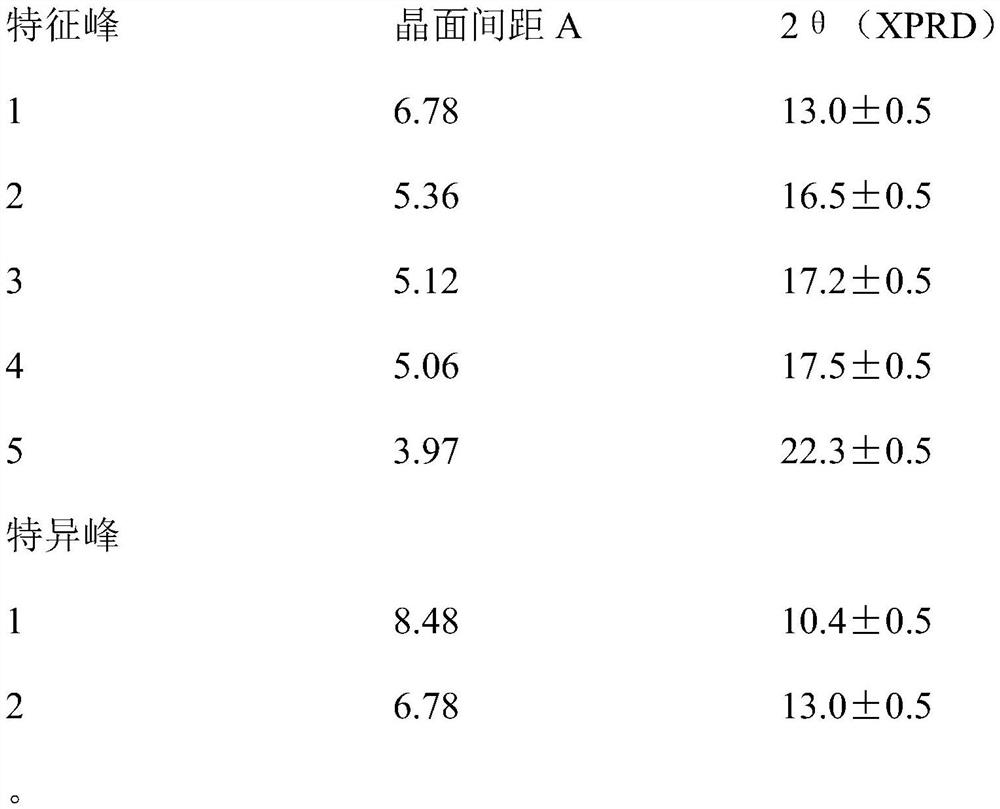
[0021] Example 1 Preparation of (1R,2S,5R)-7-oxo-6-sulfonyloxy-1,6-diazabicyclo[3,2,1﹞octane-2-carboxamide sodium salt
[0022] Add 2.25L absolute ethanol and 300g (1R,2S,5R)-7-oxo-6-sulfonyloxy-1,6-diazabicyclo﹝3,2,1﹞octane to a 5L reaction flask -Tributylbenzyl ammonium salt of 2-carboxamide, stir to dissolve, filter, heat the filtrate to 35-40°C, add 1.68L absolute ethanol, 120ml purified water, 184.4g ethylhexyl dropwise at 35-40°C Sodium acid solution. After the dropwise addition, a solid was precipitated, then the temperature was lowered to 15-25° C., stirring and crystallization was continued for 4 h, and a white solid was obtained by filtration and drying, with a yield of 70-80%.
[0023] Record the X-ray diffraction pattern in the attached figure 1 middle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com