Joint injection preparation based on collagen binding polypeptide-modified hyaluronic acid as well as preparation method and application of joint injection preparation
A hyaluronic acid and peptide-binding technology, which is applied in pharmaceutical formulations, medical science, prostheses, etc., can solve the problems of multiple injections and repeated administration, joint injection preparations and damaged cartilage surfaces cannot be combined, and achieve good resistance Non-specific adsorption properties, effects of overcoming insufficient lubricating properties, good feasibility and practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
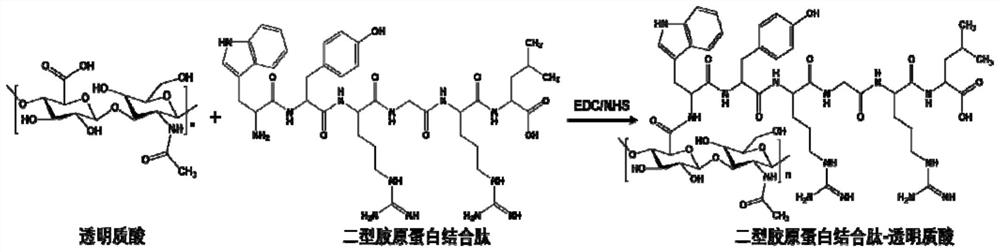
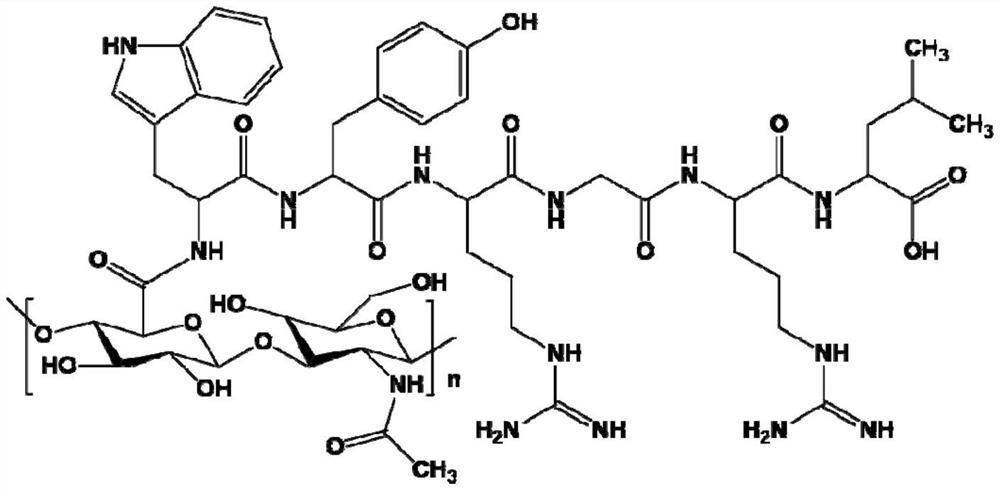
[0034] 1) Preparation of collagen-binding polypeptide-modified hyaluronic acid:
[0035] Prepare a 10 mmol / L phosphate buffer solution with pH=7.4 with disodium hydrogen phosphate dodecahydrate and potassium dihydrogen phosphate. Dissolve 1.2g of hyaluronic acid (HA) in 120mL of the above phosphate buffer, adjust the pH of the solution to 5 with 1.0mol / L hydrochloric acid, and then add 1-ethyl-(3-dimethylamino Propyl) carbodiimide hydrochloride (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and hyaluronic acid repeating unit molar ratio is 1:1), after stirring for 20min Collagen-binding polypeptides were added (the molar ratio of collagen-binding polypeptides to hyaluronic acid repeating units was 1:1), the pH of the solution was maintained at 5 with 1 mol / L hydrochloric acid, and the reaction was stirred at 20° C. for 1 hour. The reacted solution was placed in a dialysis bag (MWCO: 8000-14000Da), and dialyzed in an aqueous solution with pH=5.0 for 2 days, and th...
Embodiment 2
[0053] 1) Preparation of collagen-binding polypeptide-modified hyaluronic acid:
[0054] Prepare a 10 mmol / L phosphate buffer solution with pH=7.4 with disodium hydrogen phosphate dodecahydrate and potassium dihydrogen phosphate. Dissolve 1.2g of hyaluronic acid (HA) in 120mL of the above phosphate buffer, adjust the pH of the solution to 5.5 with 1.0mol / L hydrochloric acid, and then add 1-ethyl-(3-dimethylamino Propyl) carbodiimide hydrochloride (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and hyaluronic acid repeating unit molar ratio is 1:1), after stirring for 25min Collagen-binding polypeptides were added (the molar ratio of collagen-binding polypeptides to hyaluronic acid repeating units was 2:1), the pH of the solution was maintained at 5.5 with 1 mol / L hydrochloric acid, and the reaction was stirred at 25°C for 2 hours. The reacted solution was placed in a dialysis bag (MWCO: 8000-14000Da), and dialyzed in an aqueous solution with pH=5.0 for 2 days, and...
Embodiment 3
[0070] 1) Preparation of collagen-binding polypeptide-modified hyaluronic acid:
[0071] Prepare a 10 mmol / L phosphate buffer solution with pH=7.4 with disodium hydrogen phosphate dodecahydrate and potassium dihydrogen phosphate. Dissolve 1.2g of hyaluronic acid (HA) in 120mL of the above phosphate buffer, adjust the pH of the solution to 6 with 1.0mol / L hydrochloric acid, and then add 1-ethyl-(3-dimethylamino Propyl) carbodiimide hydrochloride (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and hyaluronic acid repeating unit molar ratio is 1:1), after stirring for 30min Collagen-binding polypeptides were added (the molar ratio of collagen-binding polypeptides to hyaluronic acid repeating units was 3:1), the pH of the solution was maintained at 6 with 1 mol / L hydrochloric acid, and the reaction was stirred at 30°C for 3 hours. The reacted solution was placed in a dialysis bag (MWCO: 8000-14000Da), and dialyzed in an aqueous solution with pH=5.0 for 2 days, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com