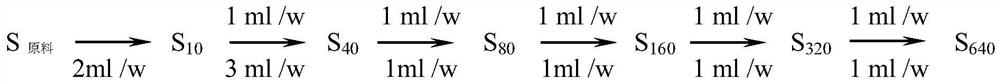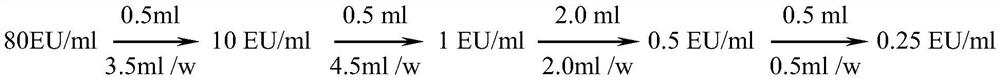Method for detecting bacterial endotoxin in nalbuphine hydrochloride bulk drug
A technology of bacterial endotoxin and nalbuphine hydrochloride, which is applied in the field of microbial inspection, can solve the problems of cumbersome and time-consuming operation of the rabbit pyrogen method, the inability to obtain quantitative results, and the inability to use quality control, etc., to reduce inspection costs and reproducibility Good, high-accuracy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention is described in further detail now in conjunction with embodiment.
[0025] 1. Experimental materials and instruments:
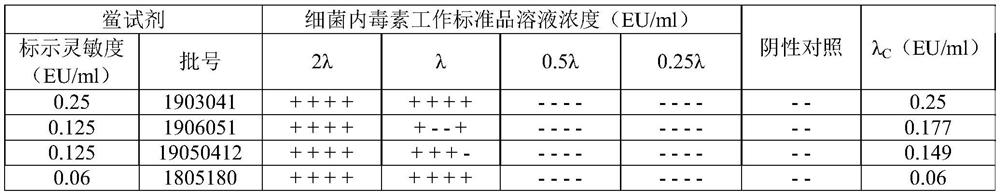
[0026] Limulus Reagent: Limulus Reagent Batch Number: 1903041, specification 0.1ml / cartridge, marked sensitivity 0.25EU / ml, produced by Zhanjiang Andus Biological Co., Ltd.; Limulus Reagent Batch Number: 1906051, specification 0.1ml / cartridge, labeled sensitivity 0.125EU / ml, Produced by Zhanjiang Andus Biological Co., Ltd.; LAL reagent lot number: 19050412, specification 0.1ml / tube, labeled sensitivity 0.125EU / ml, produced by Fuzhou Xinbei Biochemical Industry Co., Ltd.; LAL reagent lot number: 1805180, specification 0.1ml / box, labeled sensitivity 0.06EU / ml, produced by Zhanjiang Andus Biological Co., Ltd.
[0027] Bacterial endotoxin test water: Specification 50mL / bottle, batch number 1904250, containing bacterial endotoxin <0.003EU / mL, produced by Zhanjiang Andus Biological Co., Ltd.
[0028] Bacterial endotoxin working stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com