Serum ALDH1B1 autoantibody quantitative detection kit and application thereof
A technology for quantitative detection of autoantibodies, applied in the field of protein marker detection, can solve problems such as lack of research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
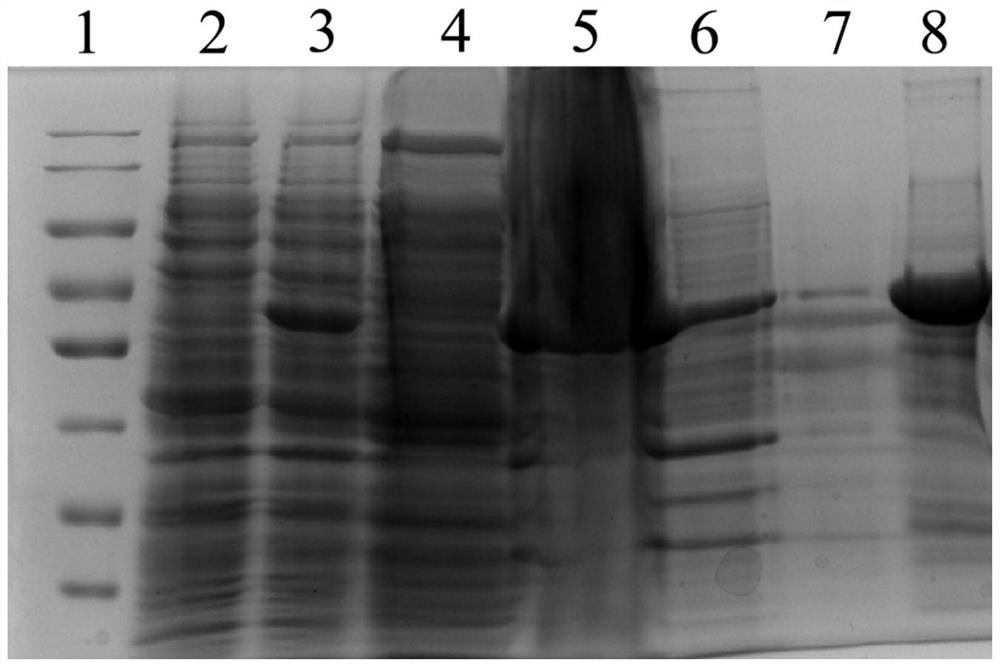
[0025] The preparation of embodiment 1 recombinant ALDH1B1 antigenic protein
[0026] 1. Materials
[0027]DNA marker (100-2000bp) and rainbow prestained protein marker (14-120KD) were purchased from Beijing Tiangen Biochemical Technology Co., Ltd.; chemiluminescent protein marker (20-90KD) was purchased from Beijing Quanshijin Biotechnology Co., Ltd.; Bacillus Origammi2 and BL21 (DE3) were preserved in our laboratory; Fast Taq Mastermix, BamHI, XhoI, and T4 DNA ligase were purchased from NEB Company; DNA electrophoresis gel recovery kit was purchased from Axygen Company; D-galactoside (IPTG) was purchased from Amresco; ALDH1B1 antibody was purchased from Beyond Biotechnology Research Institute; HRP-labeled goat anti-human IgG was purchased from Earthox, USA; primer synthesis and DNA sequence determination were provided by Shanghai Sangon Bioengineering Co., Ltd. Finish. All other reagents were of domestic analytical grade.
[0028] 2. Construction and identification of hum...
Embodiment 2
[0035] The preparation of embodiment 2 standard substance and microtiter plate
[0036] 1. Materials
[0037] The 96-well ELISA plate was purchased from Thermo Company in the United States; the recombinant ALDH1B1 antigen protein was prepared according to the method in Example 1; BSA was purchased from Beijing Huanya Tech Biomedical Technology Co., Ltd.; TMB chromogenic solution and stop solution were purchased from Beijing Solai Bao Technology Co., Ltd.; HRP-labeled rabbit anti-human IgG was purchased from Sigma, USA; other reagents were domestic analytical grade.
[0038] 2. Preparation of standard products in the detection kit
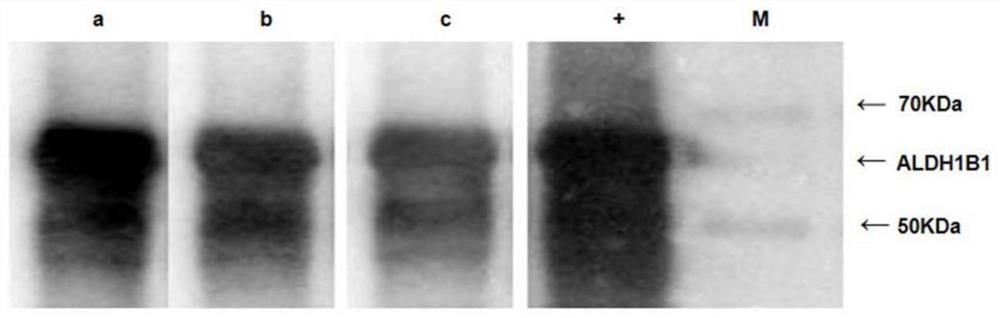
[0039] The rabbit polyclonal antibody against human ALDH1B1 was obtained by immunizing rabbits with the recombinant ALDH1B1 antigen protein prepared in Example 1, and the obtained serum antibody was further purified by affinity chromatography and used as the quantitative standard of the kit. In addition, referring to the method of Zhang Jinlong et...
Embodiment 3
[0054] Example 3 Diagnostic value of ALDH1B1 autoantibodies in colorectal cancer and colorectal high-grade adenoma
[0055] ALDH1B1 autoantibody was detected on 315 samples, including 110 healthy samples, 75 colorectal high-grade adenomas and 130 colorectal cancer serum samples, using the kit method, and the diagnostic value was evaluated by ROC curve and AUC value.
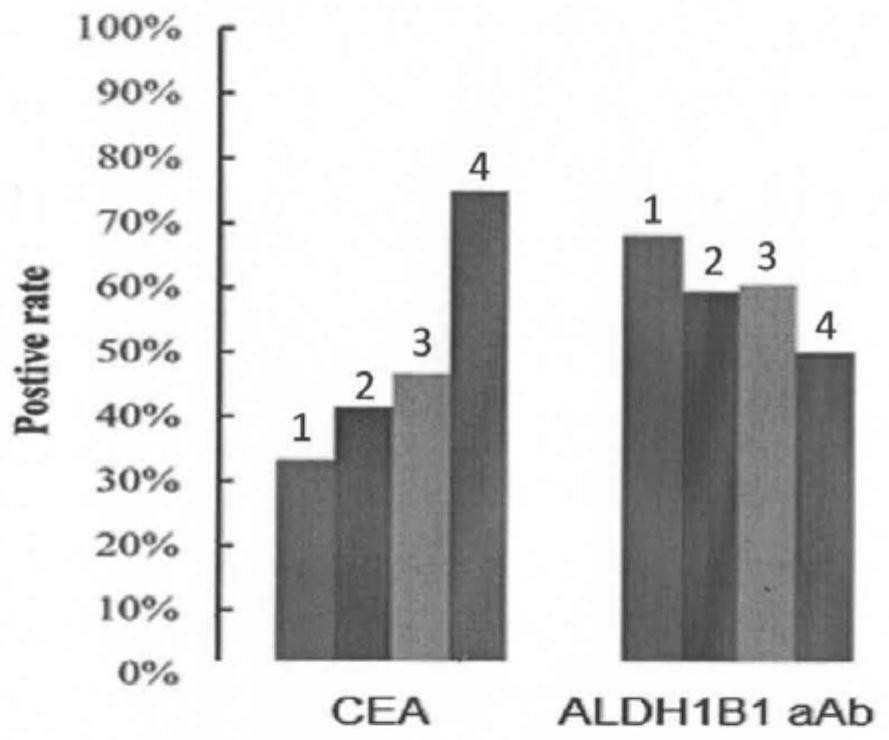
[0056] The results showed that the AUC values of ALDH1B1 autoantibody in colorectal high-grade adenoma and colorectal cancer were 0.74 and 0.70, and the sensitivity and specificity were 75.68%, 62.31%, 63.06%, and 73.87%, respectively; The positive rate of early colorectal cancer was only 38.6%, and the positive rate of ALDH1B1 was 62.3%. More than half of CEA-negative early colorectal cancer were positive for ALDH1B1 autoantibody. Experimental results such as image 3 and Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com