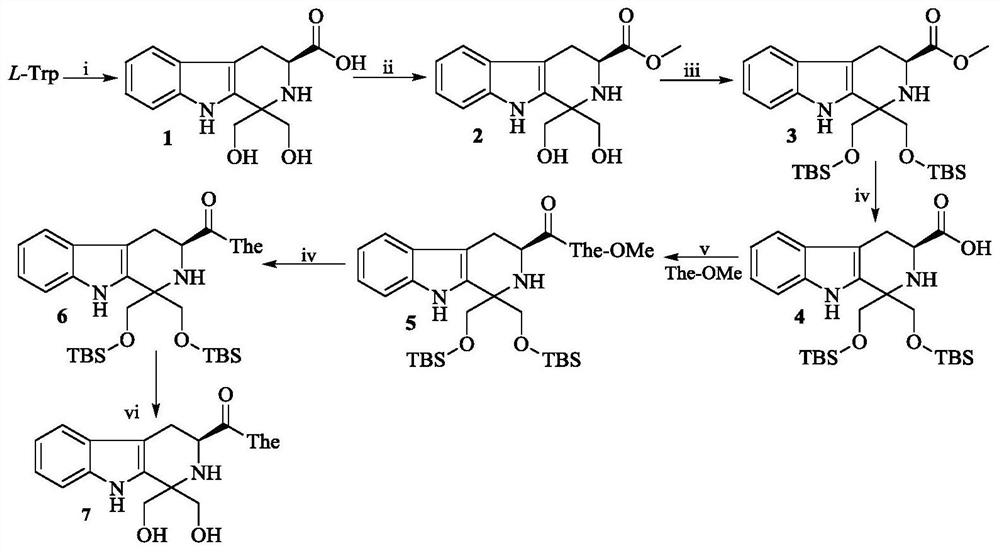
Dihydroxymethyl tetrahydrocarboline-3-formyl-The as well as synthesis, activity and application thereof
A kind of dimethylol, tert-butyldimethylsiloxy technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Preparation of (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid (1)
[0018] Add 3.06g (15mmol) of L-tryptophan to 50mL of distilled water, stir well to make it suspend. Under the condition of ice bath, concentrated sulfuric acid was slowly added dropwise to the suspension until the L-tryptophan was completely dissolved. Then add 1.62g (18mmol) 1,3-dihydroxyacetone to the solution, and react at room temperature for 84 hours. TLC showed the disappearance of L-tryptophan (ethyl acetate / water / glacial acetic acid, 4 / 1 / 1). Filtration and washing of the filter cake with ice water gave 2.90 g (70%) of the title compound as a yellow powder.
Embodiment 2
[0019] Example 2 Preparation of (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid methyl ester (2)
[0020] Slowly add 4.7mL of thionyl chloride dropwise to 50mL of methanol in an ice bath, stir for 30 minutes, then add 5.0g (18mmol) of (3S)-1,1-dimethylol-tetrahydro-β-carboline- 3-Carboxylic acid (1). Stir until compound 1 is completely dissolved. Thereafter, it was stirred at room temperature for 10 hours. TLC showed disappearance of compound 1 (dichloromethane / methanol, 20:1). The reaction mixture was concentrated under reduced pressure, and the residue was triturated with ethyl acetate. The supernatant was removed, and the residue was triturated with ethyl acetate. The resulting tan solid was dissolved in 100 mL of ethyl acetate. The solution was successively washed with saturated NaHCO 3 aqueous solution (30 mL×3), washed with saturated NaCl aqueous solution (30 mL×3), and the ethyl acetate layer was dried over anhydrous sodium sulfate for 12 hours. Filt...
Embodiment 3
[0021] Example 3 Preparation of (3S)-1,1-bis(tert-butyldimethylsilyloxy)methyl-tetrahydro-β-carboline-3-carboxylic acid methyl ester (3)
[0022] Add 4.35 g (15 mmol) of 1,1-dimethylol-β-carboline-3-carboxylic acid methyl ester (2) to 50 mL of anhydrous N,N-dimethylformamide (DMF). Stir until compound 2 is completely dissolved. Thereafter, 3.66 g (64.8 mmol) of imidazole was added to the solution under ice cooling. Stir until the imidazole is completely dissolved. Thereafter, 6.79 g (45 mmol) of tert-butyldimethylsilyl chloride (TBDMSCl) was added to the solution. The reaction mixture was stirred at room temperature for 6 hours. TLC showed that compound 2 disappeared (petroleum ether / ethyl acetate, 20 / 1). The reaction mixture was concentrated under reduced pressure, and the residue was diluted with 100 mL of saturated NaCl aqueous solution. The resulting solution was extracted with ethyl acetate (60mL×3), and the ethyl acetate layer was washed with saturated NaHCO 3 aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com