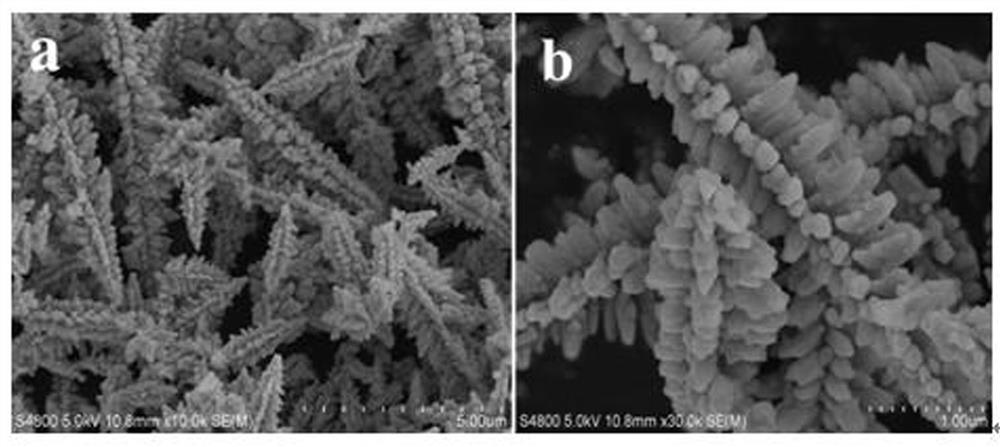
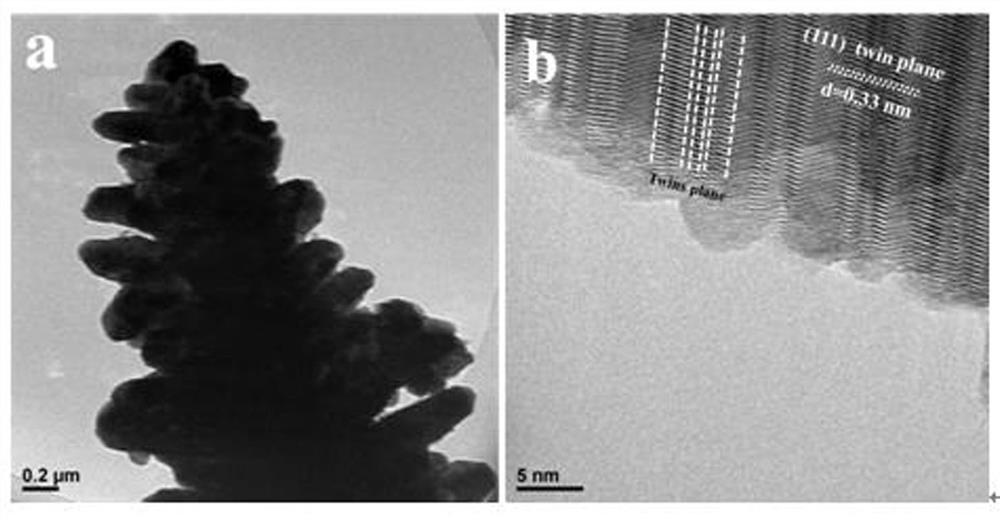
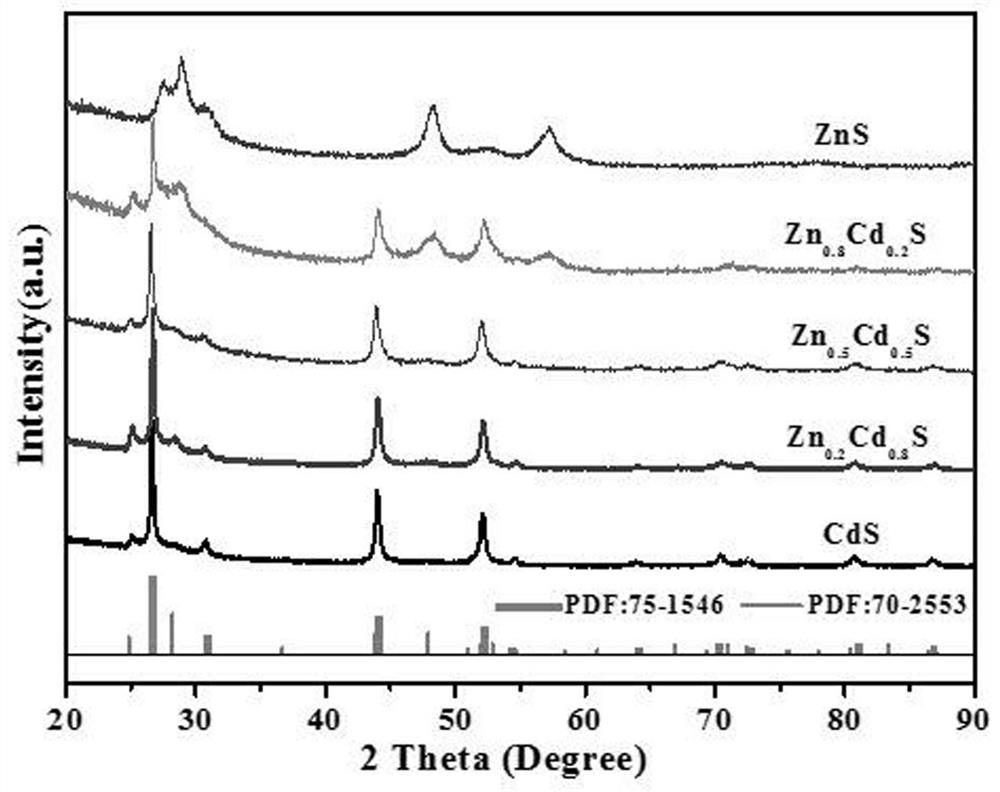
Preparation method of flower-like twin crystal phase Zn<0.2>Cd<0.8>S photocatalytic material
A photocatalytic material, twinning technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A flower-like twin phase Zn 0.2 Cd 0.8 The preparation method of S photocatalyst material, comprises the following steps:
[0032] 1) Add 0.1mmol Zn(NO 3 ) 2 ·6H 2 O and 0.9mmol Cd(NO 3 ) 2 4H 2 O was sequentially added to 60 mL deionized water, stirred and dissolved until clear and transparent to obtain a mixed solution; 2) Add 3 mmol CH 4 N 2 S was added to the mixture and kept stirring for 5 minutes until CH 4 N 2 S was completely dissolved until clear and transparent, then transferred to the reactor and added 20ml of water to the reactor for reaction, and kept at 180°C for 4 hours; 3) The product produced after the reaction was a yellow precipitate, and the obtained yellow precipitate was Separation by centrifugation, and then alternately use distilled water and ethanol to clean and wash the product; 4) After washing, the product is dried in an oven at 80°C, and the flower-like twin phase Zn is obtained after drying. 0.2 Cd 0.8 S photocatalytic material,...
Embodiment 2
[0035] A flower-like twin phase Zn 0.2 Cd 0.8 The preparation method of S photocatalyst material, comprises the following steps:
[0036] 1) Add 0.2mmol Zn(NO 3 ) 2 ·6H 2 O and 0.8mmol Cd(NO 3 ) 2 4H 2 O was sequentially added to 60 mL deionized water, stirred and dissolved until clear and transparent to obtain a mixed solution; 2) Add 3 mmol CH 4 N 2 S was added to the mixture and kept stirring for 5 minutes until CH 4 N 2 S was completely dissolved until clear and transparent, then transferred to the reactor and added 20ml of water to the reactor for reaction, and kept at 180°C for 4 hours; 3) The product produced after the reaction was a yellow precipitate, and the obtained yellow precipitate was Separation by centrifugation, and then alternately use distilled water and ethanol to clean and wash the product; 4) After washing, the product is dried in an oven at 80°C, and the flower-like twin phase Zn is obtained after drying. 0.2 Cd 0.8 S photocatalytic material,...
Embodiment 3
[0039] A flower-like twin phase Zn 0.2 Cd 0.8 The preparation method of S photocatalyst material, comprises the following steps:
[0040] 1) Add 0.2mmol Zn(NO 3 ) 2 ·6H 2 O and 0.8mmol Cd(NO 3 ) 2 4H 2 O was sequentially added to 60 mL deionized water, stirred and dissolved until clear and transparent to obtain a mixed solution; 2) Add 4 mmol CH 4 N 2 S was added to the mixture and kept stirring for 5 minutes until CH 4 N 2 S was completely dissolved until clear and transparent, then transferred to the reactor and added 20ml of water to the reactor for reaction, and kept at 180°C for 4 hours; 3) The product produced after the reaction was a yellow precipitate, and the obtained yellow precipitate was Separation by centrifugation, and then alternately use distilled water and ethanol to clean and wash the product; 4) After washing, the product is dried in an oven at 80°C, and the flower-like twin phase Zn is obtained after drying. 0.2 Cd 0.8 S photocatalytic material,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com