Electrochemical fluorination external circulation electrolysis system
An electrolysis system and external circulation technology, which is applied in the field of electrochemical fluorination external circulation electrolysis system and electrolysis system, can solve the problems of cathode gas and anode gas explosion, low efficiency, and non-circulation of electrolyte, etc., to improve energy efficiency ratio and improve Recycling efficiency and effect of miniaturization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: The system implements forced circulation
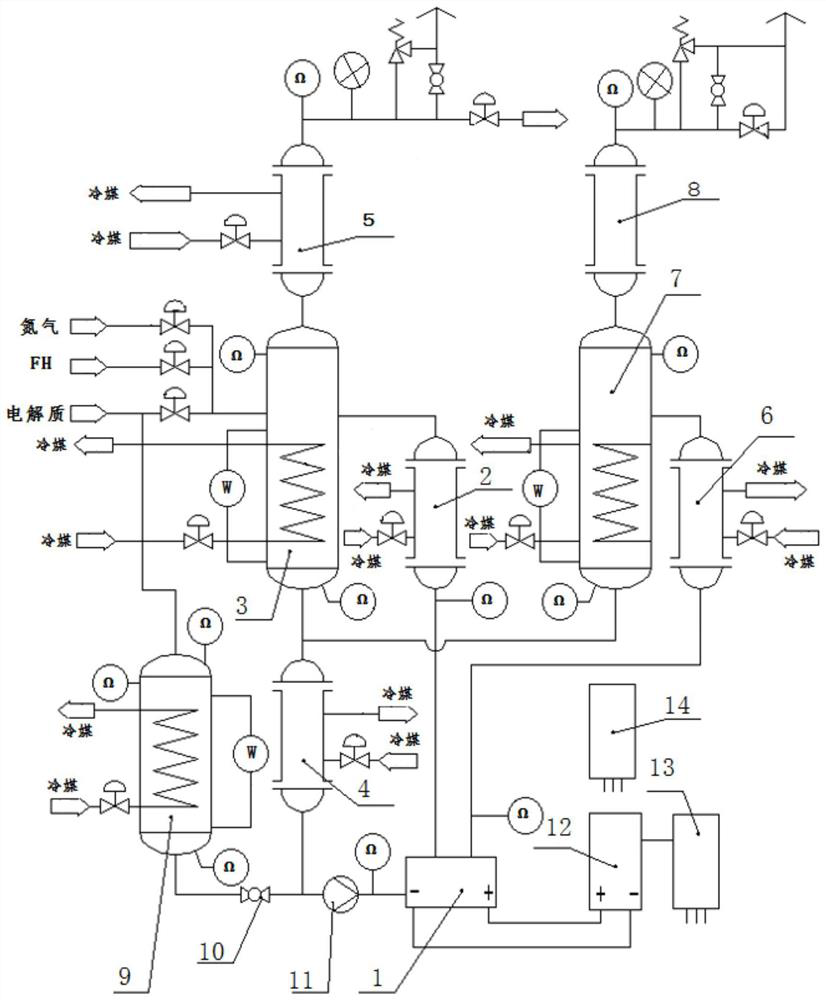
[0026]The control cabinet 14 is started, and the electrolyte and hydrogen fluoride enter the anode gas-liquid separator 3 through the control valve at a certain ratio, and continue to feed until the electrolyte level reaches the third point of the remote magnetic flap liquid level gauge in the anode gas-liquid separator 3 In one place, feeding is stopped, the circulation pump 11 is started after the nitrogen purge of the electrolysis system, and the rectifier cabinet 12 is started to transmit electricity. The anode gas and cathode gas respectively enter the anode cooler 2 and the cathode cooler 6 entrained with the electrolyte, and enter the anode gas-liquid separator 3 and the cathode gas-liquid separator 7 respectively after preliminary heat exchange. The gas phase in the anode gas and cathode gas rises into the anode condenser 5 and the cathode condenser 8, and after fully recovering the carried liquid components,...
Embodiment 2
[0027] Example 2: The system implements natural circulation
[0028] The control cabinet 14 is started, and the electrolyte and hydrogen fluoride enter the anode gas-liquid separator 3 through the control valve at a certain ratio, and continuously feed the electrolyte liquid level to the remote magnetic flap liquid level gauge in the anode gas-liquid separator 3. In one place, feed feeding is stopped, and after the electrolysis system is purged with nitrogen, the rectifier cabinet 12 starts power transmission, and under the action of the DC power supply, the cathode and anode reactions occur in the electrolysis cell 1 . The anode gas and cathode gas respectively enter the anode cooler 2 and the cathode cooler 6 entrained with the electrolyte, and enter the anode gas-liquid separator 3 and the cathode gas-liquid separator 7 respectively after preliminary heat exchange. The gas phase in the anode gas and cathode gas rises into the anode condenser 5 and the cathode condenser 8, a...
Embodiment 3
[0030] The cathode gas is directly discharged into the waste gas treatment tower without recovery, and the cathode cooler 6, cathode gas-liquid separator 7 and cathode condenser 8 can be removed from the entire electrolysis system. The control cabinet 14 is started, and the electrolyte and hydrogen fluoride enter the anode gas-liquid separator 3 through the control valve at a certain ratio, and continuously feed the electrolyte liquid level to the remote magnetic flap liquid level gauge in the anode gas-liquid separator 3. In one place, feeding is stopped, the circulation pump 11 is started after the nitrogen purge of the electrolysis system, and the rectifier cabinet 12 is started to transmit electricity. The anode gas engulfs the electrolyte and enters the anode cooler 2, and enters the anode gas-liquid separator 3 after preliminary heat exchange. The gas phase in the anode gas rises into the anode condenser 5, and after fully recovering the carried liquid components, it flo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com