Preparation method of Cu2O-PtCu catalyst and application of Cu2O-PtCu catalyst in sodium borohydride electrooxidation
A catalyst, h2ptcl6 technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of poor stability of Pt-based catalysts, achieve good catalytic activity and stability, good development prospects, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
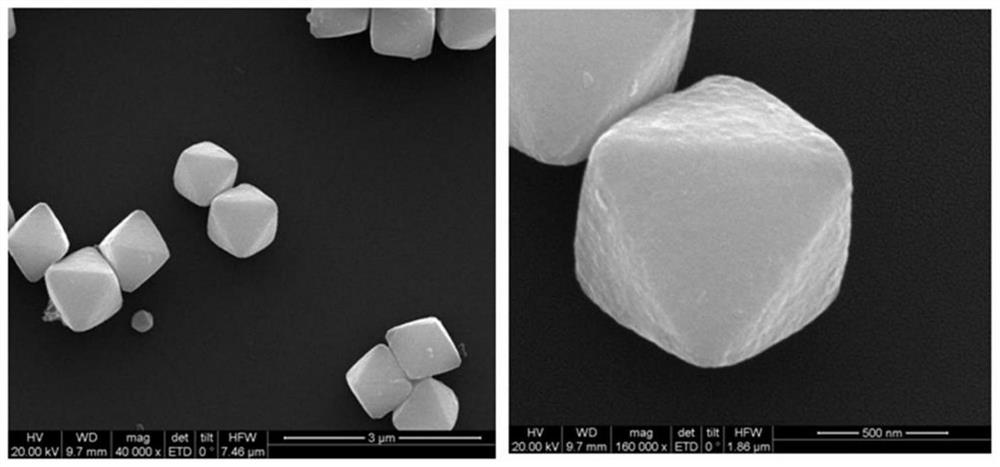
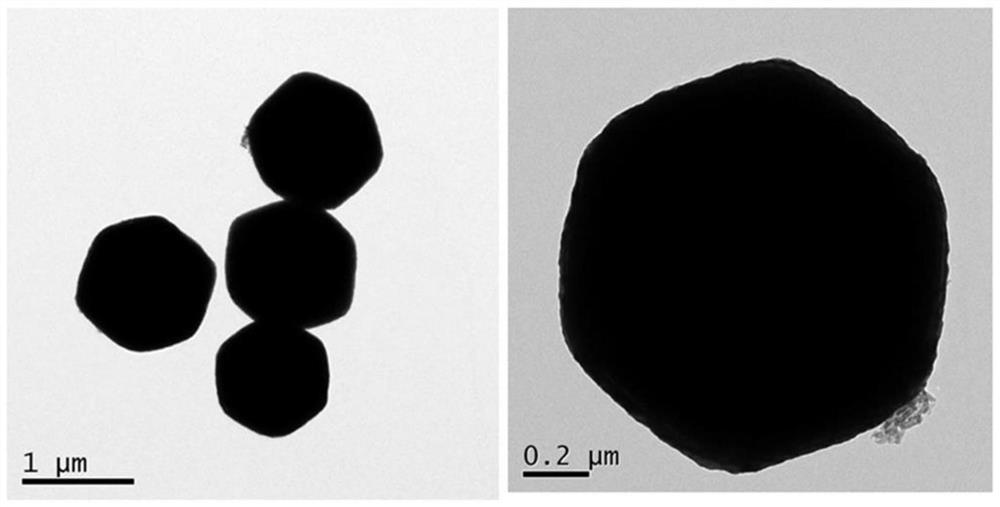
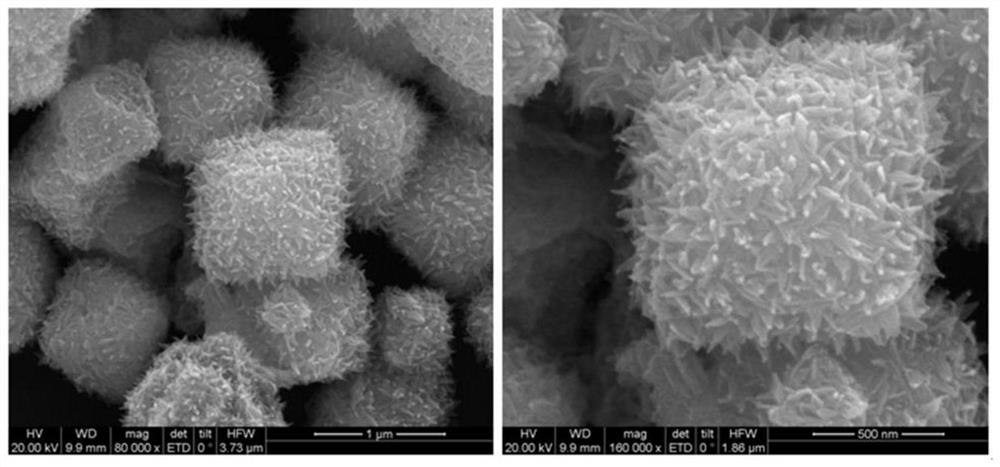
[0027] A Cu core-shell structure for sodium borohydride electrooxidation 2 The preparation method of O@PtCu catalyst, the method is prepared by the method of electro-displacement reaction, and the specific steps are as follows:
[0028] 1) Weigh 40 mg of pre-synthesized Cu 2 O octahedral powder was dissolved in 40 mL of water, 270 mg of polyvinylpyrrolidone (PVP) was added, the resulting mixture was ultrasonically treated for 10 min, and stirred for 5 min to disperse the solution evenly;
[0029] 2) Add different volumes of H under stirring condition 2 PtCl 6 (20mM) aqueous solution, reacted for 30min, then added 60μL of 36% acetic acid, continued to react for 60min under stirring conditions, centrifuged the resulting solution, washed with distilled water and absolute ethanol for 3 times, and finally dried at 40°C under vacuum for 12h, namely The target product was obtained.
[0030] The different volumes of H 2 PtCl 6 The aqueous solutions were 13.88, 4.63, 2.78, 1.98 a...
Embodiment 1
[0032] 1) Weigh 40 mg of pre-synthesized Cu 2 O octahedral powder was dissolved in 40 mL of water, 270 mg of polyvinylpyrrolidone (PVP) was added, the resulting mixture was ultrasonically treated for 10 min, and stirred for 5 min to disperse the solution evenly;
[0033] 2) Add 13.88mL of H to the mixture obtained in 1) with stirring 2 PtCl 6 (20mM) aqueous solution, reacted for 30min, then added 60μL of 36% acetic acid, continued to react for 60min under stirring conditions, centrifuged the obtained solution, washed with distilled water and ethanol for 3 times, and then dried at 40°C for 12h under vacuum condition to prepare The target product was obtained, and the obtained catalyst was denoted as Cu 2 O@PtCu-1.
[0034] The prepared catalyst is prepared as an electrode, placed in 10mmol / L NaBH 4 Electrochemical performance test in +0.1mol / LNaOH solution to study the reaction of catalyst to NaBH 4 Catalytic performance in electrooxidation.
Embodiment 2
[0036] With the step 2 in embodiment 1) in H 2 PtCl 6 The add-on of aqueous solution is changed into 4.63mL, and other steps are with embodiment 1, and the catalyst obtained is recorded as Cu2 O@PtCu-2. The catalytic performance research of the electrocatalytic sodium borohydride oxidation is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com