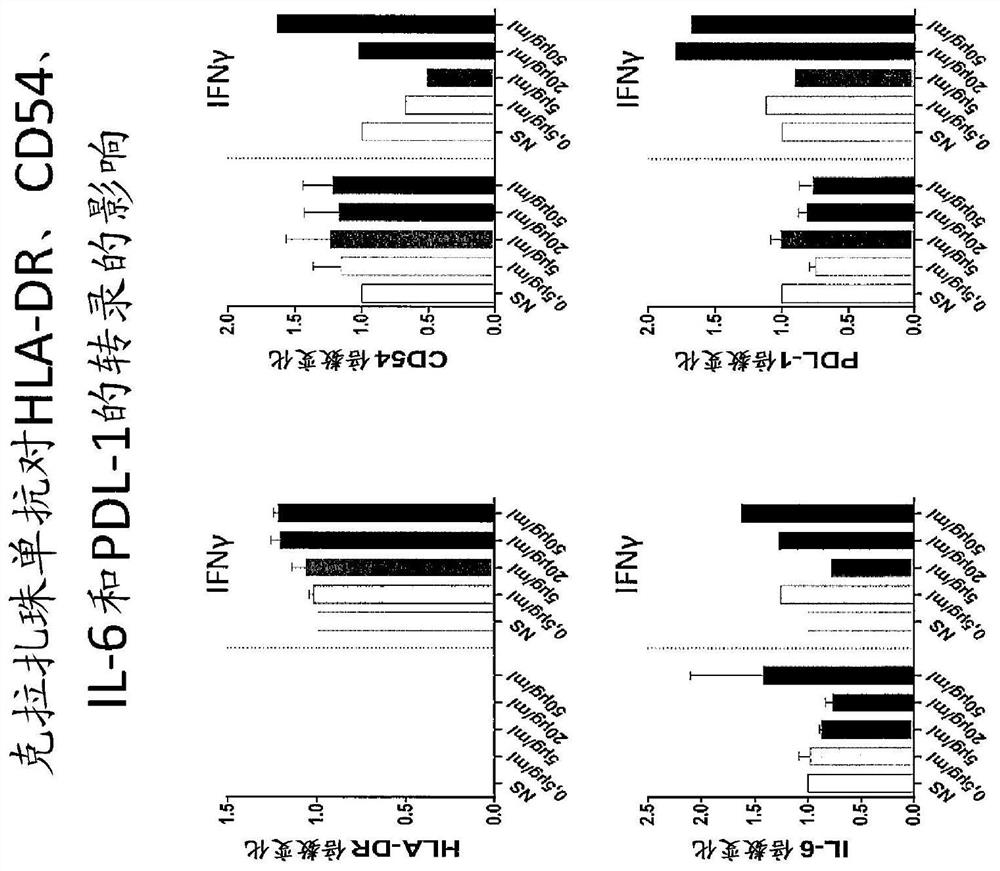
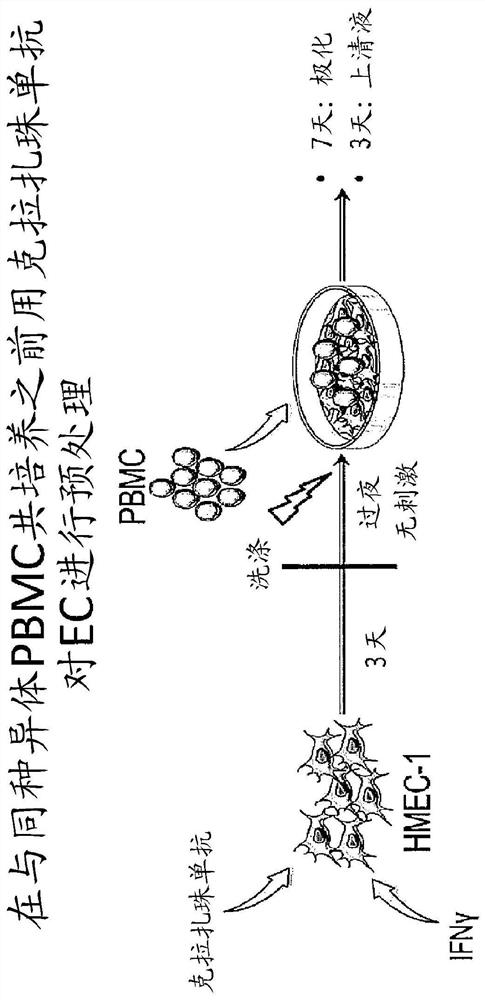
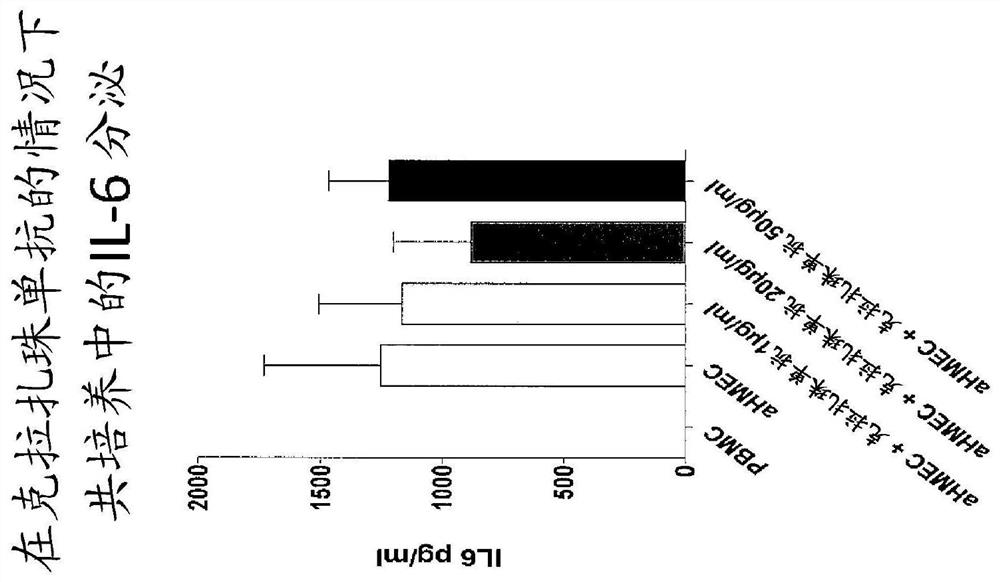
Use of Anti-il-6 antibody, e.g., clazakizumab for desensitization of solid organ transplant recipients and/or for preventing, stabilizing or reducing antibody mediated rejection (ABMR)
An IL-6, recipient technology, applied in the direction of resistance to vector-borne diseases, anti-animal/human immunoglobulins, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] Clarizumab as part of a desensitization regimen waiting for transplant and in allograft transplantation highly sensitized subjects after
[0250] Curative or prophylactic treatment to alleviate or eliminate or prevent renal transplantation in patients awaiting kidney transplantation who have previously become sensitized or are at risk of becoming sensitized to a donor organ (eg, due to history of blood transfusion, pregnancy, or previous transplantation) Sensitization to antigens (eg, HLA antigens and non-HLA antigens) present in donor organs. For example, the patient is treated by one or more of: plasmapheresis, plasma exchange, optionally with intravenous immunoglobulin and an anti-B cell agent such as rituximab or a plasma cell inhibitor such as boron Tezomib (proteosome inhibitor) combination. These procedures are repeated as necessary and are generally continued until, and may be continued after, organ transplantation.
[0251] In addition, to enhance the eff...
Embodiment 2
[0257] Example 2: Use of clarizumab in highly HLA-sensitized patients awaiting renal transplantation
[0258] Patients who have suffered previous allograft failure represent a major problem for transplant centers because they are highly human leukocyte antigen (HLA) sensitized and unlikely to receive another transplant without significant desensitization. Eligible transplant patients in accordance with the present invention will typically receive up to 6 once-monthly doses of clarizumab at 25 mg prior to transplantation. If the patient received an HLAi transplant during treatment, participants could continue to receive 6 additional once-monthly doses of clarizumab at a dose of 25 mg, followed by a 6-month regimen of biopsies. If improvement is observed after the 6th dose of clarizumab, patients will receive 6 additional doses over 6 months. Patients showing signs of persistent allograft dysfunction may undergo causative off-protocol biopsy. Patients receiving 12 doses of c...
Embodiment 3
[0262] Example 3: Treatment of patients with advanced AMBR with clarizumab
[0263] Evaluation of the safety, tolerability, pharmacokinetics, pharmacokinetics, and Pharmacodynamics and efficacy (initial assessment). The study was designed as a phase 2 trial and had two follow-up subparts, a 12-week randomized placebo-controlled trial (Part A), in which recipients were assigned to receive the anti-IL-6 antibody clarizumab (n =10) or placebo (n=10), followed by an open-label prospective study in which all 20 study patients received clarizumab for a 40-week period. A study protocol biopsy was performed at the end of Part A and Part B.
[0264] Part A:
[0265] Patients who were positive for anti-HLA donor-specific antibodies (DSA) and had biopsy-proven advanced ABMR (acute / active or chronic / active according to Banff 2015 classification) were identified and recruited at the Kidney Transplant Outpatient Service at two central sites. sexual phenotype) patients. Participants w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com