Alpha-glucosidase inhibitor and application thereof
A technology of glucosidase and inhibitor, which is applied in the field of medicine to achieve the effects of inhibitory activity, low toxicity and enhanced inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
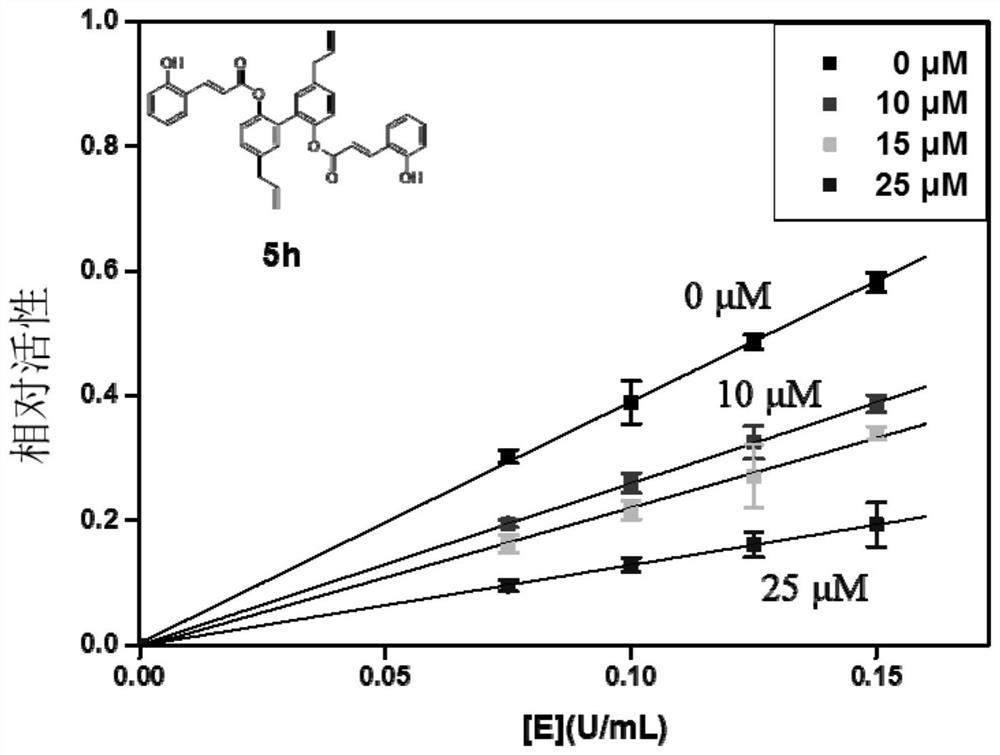
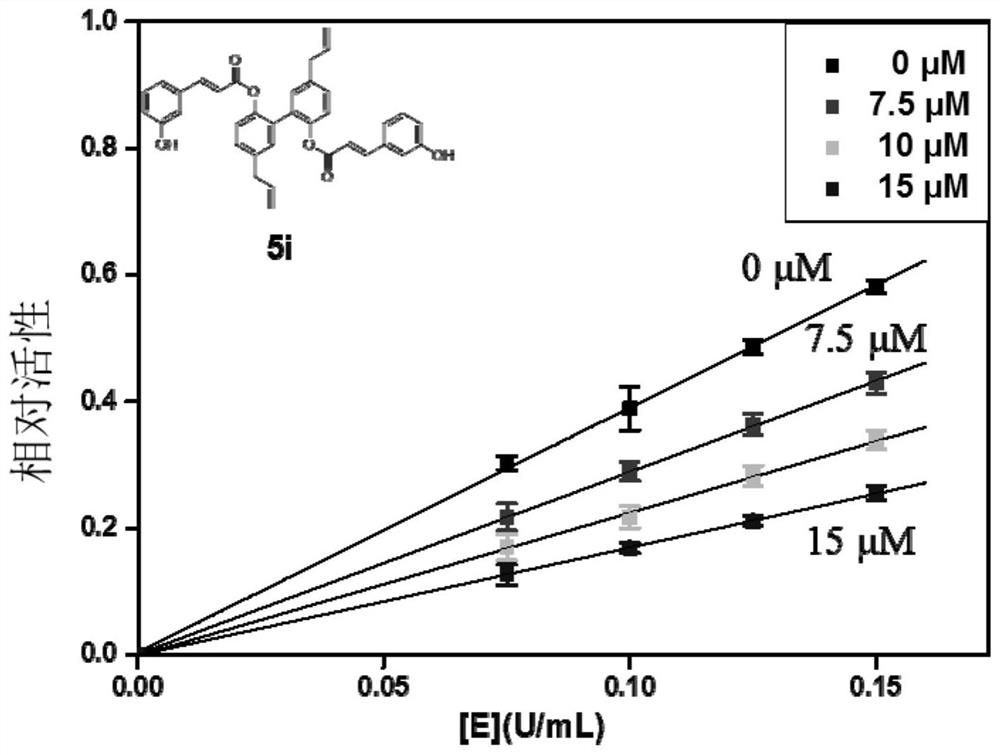
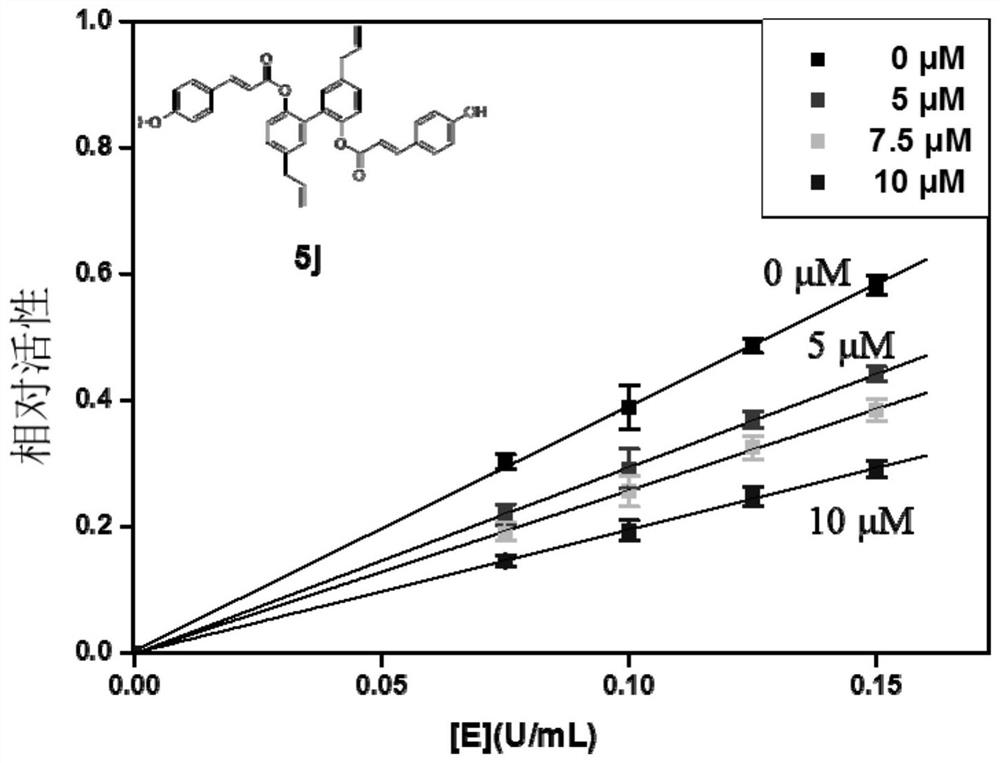
[0046] Embodiment 1: α-glucosidase inhibitor and its preparation
[0047] The α-glucosidase inhibitors in the embodiments of the present invention are all cinnamic acid-magnolol derivatives, and the structural formula of cinnamic acid-magnolol derivatives is:
[0048]
[0049] Among them, R 1 independently selected from a hydrogen atom, an alkyl group, an alkoxy group, a halogen, a haloalkyl group or a hydroxyl group; R 1 Located in the ortho, meta or para position of the benzene ring.
[0050] Specifically, in the embodiments of the present invention, the compounds listed in the following Table 1 are included (respectively denoted as 4a~4j and 5a~5j):
[0051] Table 1. Cinnamic Acid-Magnolol Derivatives
[0052]
[0053] In the present invention, "2-" corresponds to the ortho position of the benzene ring, "3-" corresponds to the meta position of the benzene ring, and "4-" corresponds to the para position of the benzene ring.
[0054] The compound of 4a~4j in table 1...
Embodiment 2
[0086] Embodiment 2: α-glucosidase inhibitory activity test of compound
[0087] 1. Microplate method to verify the inhibitory activity of α-glucosidase in vitro:
[0088] 1.1 Principle: α-glucosidase catalyzes the hydrolysis of 4-nitrophenyl-α-D-glucopyranoside (PNPG) to produce nitrophenol (PNP, yellow substance with maximum absorption at 405nm), α-glucosidase The inhibitor can inhibit the combination of α-glucosidase and the substrate to reduce the release of PNP, and the enzyme inhibitory activity of the extract is calculated by the change of PNP content in the reaction system within a certain period of time.
[0089] (1) Preparation of 100mM phosphate buffer solution (PBS) with pH 6.8: Mix and dissolve 17.907g of disodium hydrogen phosphate and 6.804g of potassium dihydrogen phosphate respectively, then add deionized water to make up to 1L, and use a pH meter to Measure the pH.
[0090] (2) Preparation of cinnamic acid-magnolol series derivative solution: Weigh a certai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com