Substituted quinoline carboxamide compound and application thereof
A compound and hydrate technology, applied in the directions of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problem of uncontrolled cell proliferation, and achieve the effect of inhibiting angiogenesis, good effect, and inhibiting cell proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
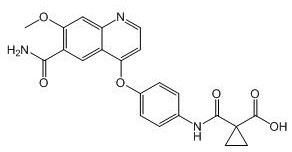
[0121] Example 1: 4-(4-((2-(cyclopropylamino)-3,4-dioxocyclobut-1-en-1-yl)amino)phenoxy)-7-methoxy Preparation of quinoline-6-carboxamide (compound 1)
[0122] (1) Preparation of intermediate 1:
[0123]
[0124]In a 50 mL flask was added 30 mL of DMSO, followed by 4-hydroxy-7-methoxyquinoline-6-carboxamide (2.2 g, 1 eq), 4-(Boc)aminophenol (2.3 g, 1.1 eq), four Butylammonium iodide (0.5eq) and cesium carbonate (3.6g, 1.1eq), the reaction solution was heated to 100°C, and reacted for 10h. Post-reaction treatment: the reaction liquid was cooled to room temperature, diluted with a large amount of water, extracted three times with ethyl acetate, the organic phase was washed with saturated brine, then dried with anhydrous sodium sulfate, and rotary evaporated to obtain 2.2 g of intermediate 1.
[0125] MS m / z 310.1 (M+1) + ;
[0126] 1 H-NMR (400MHz, CDCl 3 ) δ: 8.68-8.64(m, 2H), 7.87-7.76(m, 2H), 6.60-6.56(m, 4H), 4.04(s, 3H).
[0127] (2) Preparation of intermediate 2:...
Embodiment 2-4
[0137] Example 2-4: 4-(4-((2-(cyclopropylamino)-3,4-dioxocyclobut-1-en-1-yl)amino)-3-fluorophenoxy) -7-methoxyquinoline-6-carboxamide (compound 2); 4-(4-((2-(cyclopropylamino)-3,4-dioxocyclobut-1-ene-1- base)amino)-3-(trifluoromethyl)phenoxy)-7-methoxyquinoline-6-carboxamide (compound 3); and 4-(4-((2-(cyclopropane (amino)-3,4-dioxocyclobut-1-en-1-yl)amino)-3-chlorophenoxy)-7-methoxyquinoline-6-carboxamide (compound a) preparation
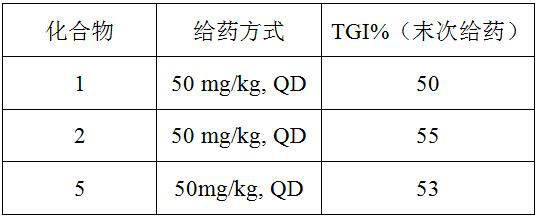
[0138] Compounds 2, 3, and a were synthesized according to the operation similar to that of Example 1 (see Table 1).
[0139] Table 1 Structure and mass spectrum data of compounds 2, 3 and a
[0140] Example compound structural formula MS (ESI) M+H 2 2 Description: Description: C:\Program Files (x86)\gwssi\CPC client\cases\inventions\00a6d582-9f9b-4bc9-b061-c25ba57eeb68\new\100002\939081dest_path_image007.jpg
Embodiment 5
[0141] Example 5: N-(4-((6-carbamoyl-7-methoxyquinolin-4-yl)oxy)phenyl)-N-cyclopropylcyclopropane-1,1-dicarboxy Preparation of acid amide (compound 4)
[0142] (1) Preparation of intermediate 3:
[0143]
[0144] To anhydrous THF (100 mL) was added 1,1-cyclopropyldicarboxylic acid (10.10 g, 70 mmol, 1.0 eq). Under nitrogen protection, triethylamine (7.03g, 70mmol, 1.0eq) was added dropwise and stirred at 0°C for 30min, followed by addition of thionyl chloride (8.26g, 70mmol, 1.0eq) and stirred at 0°C for 30min minute. Under nitrogen protection, a solution of intermediate 1 (23.7 g, 77 mmol, 1.1 eq) in anhydrous THF (50 mL) was added dropwise to the reaction solution, and stirred at 0° C. for 1.5 h. The reaction solution was diluted with ethyl acetate and extracted with 2N NaOH (pH>10). The aqueous phase was added dropwise with 2N HCl to adjust the pH to 1-2, and then extracted with ethyl acetate. The organic phase was dried and suspended to obtain 11.3 g of intermediat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com