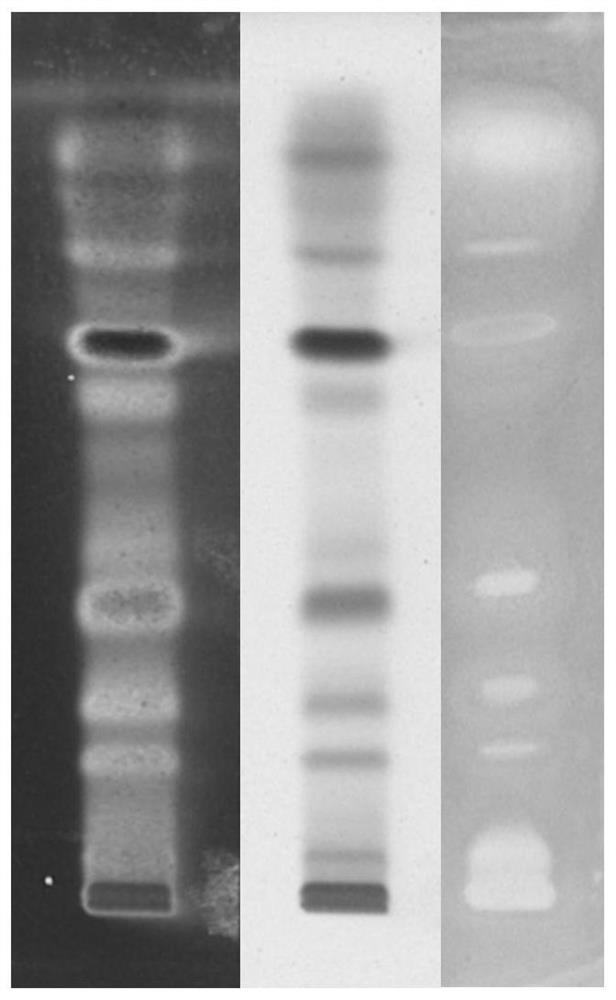
Method for screening xanthine oxidase inhibitor
A technology of xanthine oxidase and screening method, which is applied in the field of screening of xanthine oxidase inhibitors, can solve problems such as inability to apply complex system specificity, and achieve the effects of low experimental cost, simple operation, and improved specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A screening method for xanthine oxidase inhibitors, the specific operations are as follows:
[0048] (1) The preparation process of various solutions is as follows:
[0049] Preparation of developer solution: Weigh 20g of sodium tungstate, dissolve it in 150mL of distilled water, add 16mL of phosphoric acid solution, heat and reflux for 2 hours, cool to room temperature, and dilute to 280mL with distilled water to make phosphotungstic acid storage solution, keep away from light For storage, dilute 1.45 times with distilled water before use;
[0050] Preparation of phosphate buffer: Accurately weigh 22.822g of dipotassium hydrogen phosphate (analytical pure), 13.609g of potassium dihydrogen phosphate (analytical pure), add them to a 500mL volumetric flask, and distilled water to make up the phosphoric acid of pH=7.1 buffer;
[0051] Preparation of xanthine oxidase solution: Dissolve EDTA disodium salt with pH=7.1 phosphate buffer solution, and finally prepare enzymatic...
Embodiment 2
[0060] A screening method for xanthine oxidase inhibitors, the specific operations are as follows:
[0061] (1) The preparation process of various solutions is as follows:
[0062] Preparation of developer solution: Weigh 20g of sodium tungstate, dissolve it in 150mL of distilled water, add 16mL of phosphoric acid solution, heat and reflux for 3 hours, cool to room temperature, and distill the volume to 280mL with distilled water to make phosphotungstic acid storage solution, keep away from light Store, dilute 1.6 times with distilled water before use;
[0063] Preparation of phosphate buffer solution: Accurately weigh 10.806g of dipotassium hydrogen phosphate (analytical pure), 2.245g of potassium dihydrogen phosphate (analytical pure), add them to a 500mL volumetric flask, and distilled water to make up the phosphoric acid of pH=7.6 buffer;
[0064] Preparation of xanthine oxidase solution: Dissolve EDTA disodium salt with pH=7.6 phosphate buffer solution, and finally prep...
Embodiment 3
[0072] A screening method for xanthine oxidase inhibitors, the specific operations are as follows:
[0073] (1) The preparation process of various solutions is as follows:
[0074] Preparation of developer solution: Weigh 20g of sodium tungstate, dissolve it in 150mL of distilled water, add 16mL of phosphoric acid solution, heat and reflux for 4 hours, cool to room temperature, and distill the volume to 280mL with distilled water to make phosphotungstic acid storage solution, keep away from light For storage, dilute 1.75 times with distilled water before use;
[0075] Preparation of phosphate buffer solution: accurately weigh 6.008g of dipotassium hydrogen phosphate (analytical pure), 3.682g of potassium dihydrogen phosphate (analytical pure), add them to a 500ml volumetric flask, distill to volume, and prepare pH=8.0 Phosphate buffer;
[0076]Preparation of xanthine oxidase solution: Dissolve EDTA disodium salt in phosphate buffer solution with pH=8.0, and finally prepare e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com