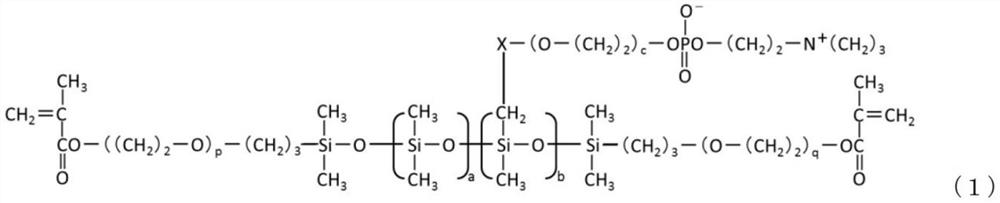
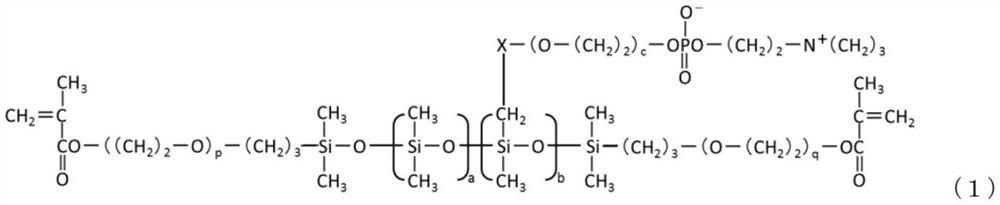
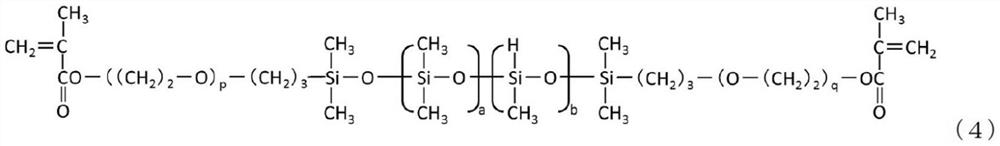
Phosphorylcholine group-containing polysiloxane monomer
A technology of polysiloxane monomer and acylcholine, which is applied in instruments, glasses/goggles, optics, etc., to achieve the effects of suitable mechanical strength, surface hydrophilicity and suitable oxygen permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] The analysis condition of embodiment 1
[0148] The analysis conditions of Example 1 are as follows.
[0149] 1 H NMR assay
[0150] Measuring device: JNM-AL400 manufactured by JEOL Ltd.
[0151] Solvent: CDCl 3 (TMS Benchmark)
Embodiment 2
[0152] Components used in Example 2 and Comparative Example
[0153] Components other than the polysiloxane monomer of the present invention used in Example 2 and Comparative Example are as follows.
[0154] 〇Comparative compound of formula (1)
[0155] PMPC: a macromonomer containing a phosphorylcholine functional group side chain (the compound of Experimental Example 4 of Patent Document 8)
[0156] FM-7721: JNC Corporation, two-terminal methacryloxy polydimethylsiloxane (molecular weight ≒ 5,000)
[0157] 〇Other monomers
[0158] SiGMA: 2-Hydroxy-3-(tris(trimethylsilyloxy)silyl)propyl methacrylate ES: 4-(2-hydroxyethyl)=1-[3-tris(trimethyl) silyloxy) silyl propyl] = 2-methine succinate
[0159] TEGDMA: Tetraethylene glycol dimethacrylate
[0160] 〇Hydrophilic monomer
[0161] MPC: 2-(methacryloyloxyethyl)-2-(trimethylaminoethyl)phosphate
[0162] HPMA: Hydroxypropyl methacrylate (a mixture of 2-hydroxypropyl and 2-hydroxy-1-methylethyl ester, manufactured by NIPPONSH...
Embodiment 1-1
[0178] In a 100 mL three-necked flask, make 10.00 g of silicone intermediate 1 (hydrosilyl group-containing two-terminal methacryloyl silicone represented by formula (4)) and 0.2140 g of alkene-containing silicone represented by formula (5) The propyl phosphorylcholine compound was dissolved in 10.00 g of 2-propanol, heated to 80° C. using an oil bath, and then 40 μL of a 4% by weight hexachloroplatinic acid hexahydrate 2-propanol solution was added.
[0179] Dissolve 1.93 g of the allyl group-containing phosphorylcholine compound represented by the formula (5) in a solution of 5.78 g of 2-propanol to obtain a solution, add the solution to a 10 mL dropping funnel, and install In the upper part of the three-neck flask.
[0180] The solution in the dropping funnel was dropped over 30 minutes while maintaining 80°C. After the dropwise addition, the reaction was further carried out in a reflux state for 1 hour. After removing low-boiling point components by vacuum distillation, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com