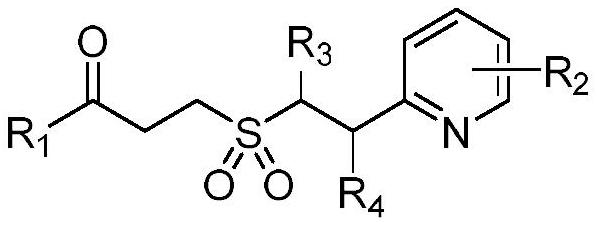
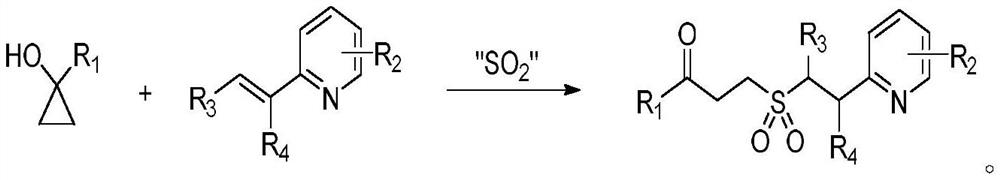
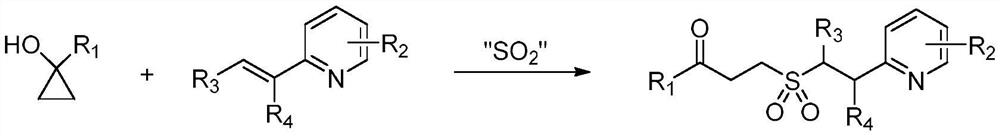
Synthesis method of gamma-keto sulfone compound
A synthesis method and compound technology, applied in the field of synthesis of γ-ketosulfone compounds, can solve problems such as limiting the scope of application of substrates, achieve good guiding significance, application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Add 0.4 mmol of 2-vinylpyridine, 0.6 mmol of 1-phenylcyclopropanol and 0.8 mmol of DABCO·(SO 2 ) 2 , seal the reaction tube with a stopper, replace the gas in high-purity nitrogen, make the system in anaerobic condition, add 2mL of acetonitrile, and stir in an oil bath at 40°C until the reaction is complete. The reaction solution was directly concentrated under reduced pressure, and separated by column chromatography, using a mixture of dichloromethane and methanol as the mobile phase to obtain the corresponding compound example 1.
[0022] Structural characterization of compound example 1: 1 H NMR (400MHz, CDCl 3 )δ8.52(d,J=3.1Hz,1H),7.97(m,2H),7.69–7.57(m,2H),7.49(m,2H),7.27(s,1H),7.18(dd,J =7.1,5.1Hz,1H),3.67–3.52(m,4H),3.44–3.32(m,4H); 13 C NMR (100MHz, CDCl 3 )δ195.66, 157.00, 149.50, 136.90, 135.76, 133.86, 128.83, 128.14, 123.48, 122.17, 52.39, 47.85, 30.84, 30.14; HRMS (ESI): m / z [M+H] + calcd for C 16 h 18 NO 3 S + :304.1007; found: 304.10...
Embodiment 2
[0024]
[0025] Add 0.2 mmol of 4-vinylpyridine, 0.3 mmol of 1-phenylcyclopropanol and 0.4 mmol of DABCO.(SO 2 ) 2 , seal the reaction tube with a stopper, replace the gas in high-purity nitrogen, make the system in anaerobic condition, add 1mL of acetonitrile, and stir in an oil bath at 40°C until the reaction is complete. The reaction solution was directly concentrated under reduced pressure, and separated by column chromatography, using a mixture of dichloromethane and methanol as the mobile phase to obtain the corresponding compound example 2.
[0026] Structural characterization of compound example 2: 1 H NMR (400MHz, CDCl 3 )δ8.54(d,J=4.2Hz,1H),7.95–7.87(m,2H),7.63–7.54(m,2H),7.46(t,J=7.7Hz,2H),7.38(s,1H ),7.37(s,1H),7.30(t,J=7.5 Hz,2H),7.22(t,J=7.1Hz,2H),7.18–7.08(m,1H),4.79(dd,J=8.1, 5.3Hz,1H),4.56(dd,J=14.6,8.2Hz,1H),3.64(dd,J=14.6,5.3Hz,1H),3.51–3.32(m,2H),3.08–2.90(m,2H ); 13 C NMR (100MHz, CDCl 3 )δ195.55, 159.52, 149.22, 141.27, 137.12, 135.80, 133.77, 1...
Embodiment 3
[0028]
[0029] Add 0.2mmol of 3-methyl-2-vinylpyridine, 0.3mmol of 1-phenylcyclopropanol and 0.4mmol of DABCO.(SO 2 ) 2 , seal the reaction tube with a stopper, replace the gas in high-purity nitrogen, make the system in anaerobic condition, add 1mL of acetonitrile, and stir in an oil bath at 40°C until the reaction is complete. The reaction solution was directly concentrated under reduced pressure, and separated by column chromatography, using a mixture of dichloromethane and methanol as the mobile phase to obtain the corresponding 1-phenyl-3-(3-methylpyridine-2-ethyl ) Example 3 of sulfonyl acetonide compound.
[0030] Structural characterization of compound example 3: 1 H NMR (400MHz, CDCl 3 )δ8.36–8.24(m,1H),8.01–7.93(m,2H), 7.65–7.57(m,1H),7.48(dd,J=16.2,8.5Hz,3H),7.09(dd,J= 7.6,4.8Hz,1H),3.69(dd,J=9.6,5.9Hz,2H),3.64–3.55(m,2H),3.45(dd,J=8.2,6.6Hz,2H),3.37–3.28(m ,2H); 13 C NMR (100MHz, CDCl 3)δ195.71, 155.10, 146.54, 137.82, 135.80, 133.84, 131.59, 128.84, 128...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com