Laingolide A and diastereoisomer and synthetic method thereof
A technology of diastereomers and synthesis methods, applied in the field of LaingolideA and its diastereomers, can solve the problem of lack of synthesis methods, and achieve the effects of clear route, great practical value and significance, and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
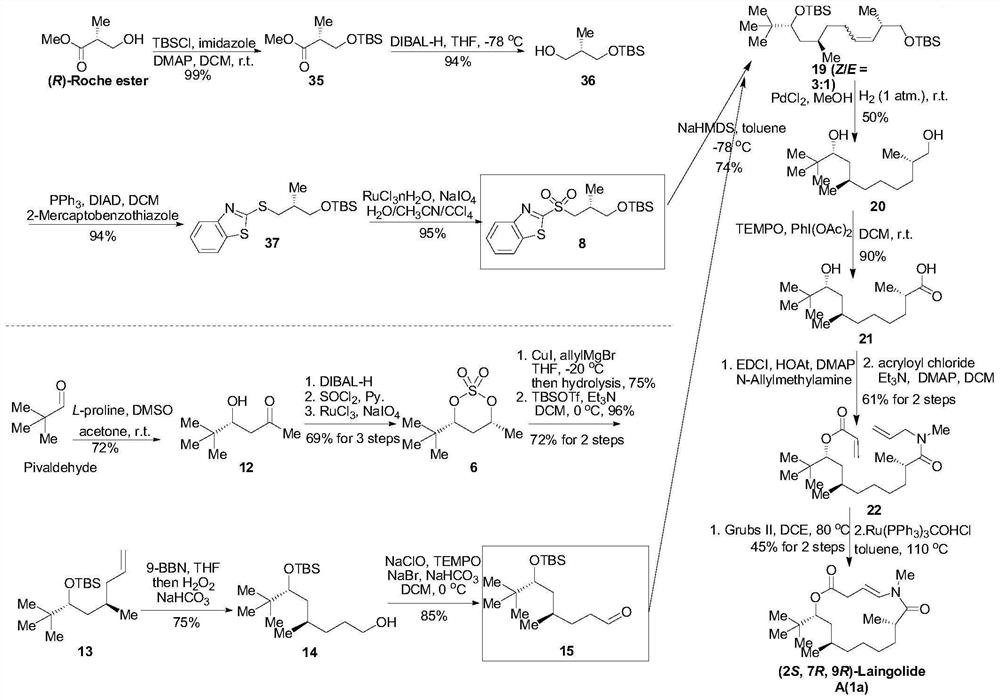
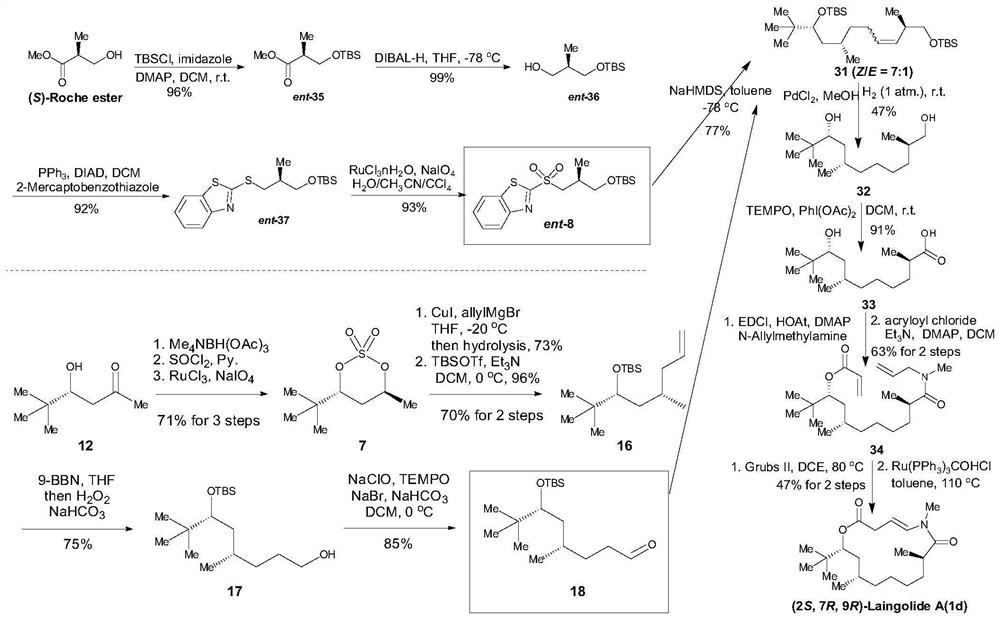
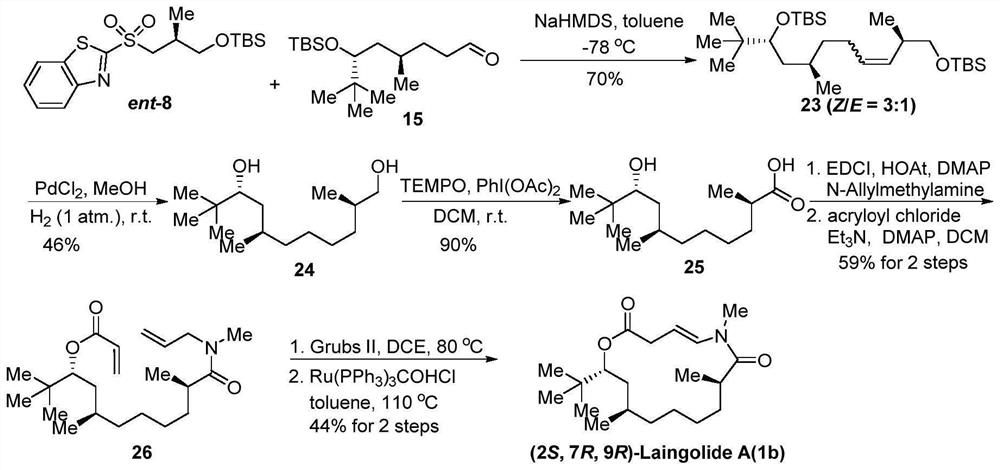
[0024] The first aspect of the embodiment of the present application provides a synthetic method of Laingolide A and its diastereoisomers, comprising steps:
[0025] S10. Obtain compound 8 or ent-8; obtain compound 15 or 18;
[0026] S20. Compound 8 or ent-8 and compound 15 or 18 are subjected to a Julia olefin synthesis reaction to obtain compound A;
[0027] S30. Reductive hydrogenation treatment is performed on compound A to obtain compound B;
[0028] S40. Oxidizing compound B to obtain compound C;
[0029] S50. performing an esterification reaction after compound C is assembled with an amide bond to obtain compound D;
[0030] S60. After compound D is subjected to olefin metathesis reaction, olefin isomerization reaction is carried out to obtain Laingolide A or the diastereoisomers of Laingolide A;
[0031] Wherein, the structural formula of compound 8 is: The structural formula of compound ent-8 is: The structural formula of compound 15 is: The structural formul...
Embodiment 1
[0090] Embodiment 1: synthetic compound 8 intermediate, design following synthetic route:
[0091]
[0092] Include the following specific steps:
[0093] 1. Synthesis of compound 35: (R)-Roche ester (1.0g, 8.5mmol, 1.0eq.), imidazole (1.2 g, 16.9mmol, 2.0eq.) and 4-dimethylaminopyridine DMAP (207.0 mg, 1.7mmol, 0.2eq.) was dissolved in dry dichloromethane DCM (40mL, 0.21M). At 0 °C, tert-butyldimethylsilyl chloride TBSCl (1.5 g, 10.2 mmol, 1.2 eq.) was added, then the reaction temperature of the reaction was raised to room temperature and stirring was continued for 16 h. After the reaction is complete, add saturated NH 4 The reaction was quenched with Cl (30 mL), then the aqueous phase was extracted with DCM (3×40 mL), the organic phases were combined and washed with excess anhydrous Na 2 SO 4 Drying, followed by filtration to obtain the filtrate containing the product, the filtrate was concentrated by a vacuum water pump to obtain a crude product, and finally the obta...
Embodiment 2
[0097] Embodiment 2: synthetic compound ent-8 intermediate, design following synthetic route:
[0098]
[0099] Include the following specific steps:
[0100] 1. Synthesis of compound ent-35: (S)-Roche ester (1.0g, 8.5mmol, 1.0eq.), imidazole (1.2g, 16.9mmol, 2.0eq.) and DMAP (207.0mg, 1.7mmol, 0.2 eq.) was dissolved in dry DCM (40mL, 0.21M), and TBSCl (1.5g, 10.2mmol, 1.2eq.) was added at 0°C, then the reaction temperature was raised to room temperature and stirring was continued for 16h. After the reaction is complete, add saturated NH 4 The reaction was quenched with Cl (30 mL), then the aqueous phase was extracted with DCM (3×40 mL), the organic phases were combined and washed with excess anhydrous Na 2 SO 4 Drying, followed by filtration to obtain a filtrate containing the product, the filtrate was concentrated by a vacuum water pump to obtain a crude product, and finally the obtained crude product was purified by column chromatography to obtain compound ent-35 (1.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com