Preparation method of R-type chiral sulfoxide compound
A compound and sulfoxide technology, applied in the field of preparation of R-type chiral sulfoxide compounds, can solve the problem of difficulty in separation, low content, affecting S-type ticagrelor sulfoxide or R-type ticagrelor sulfoxide process and other issues, to achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
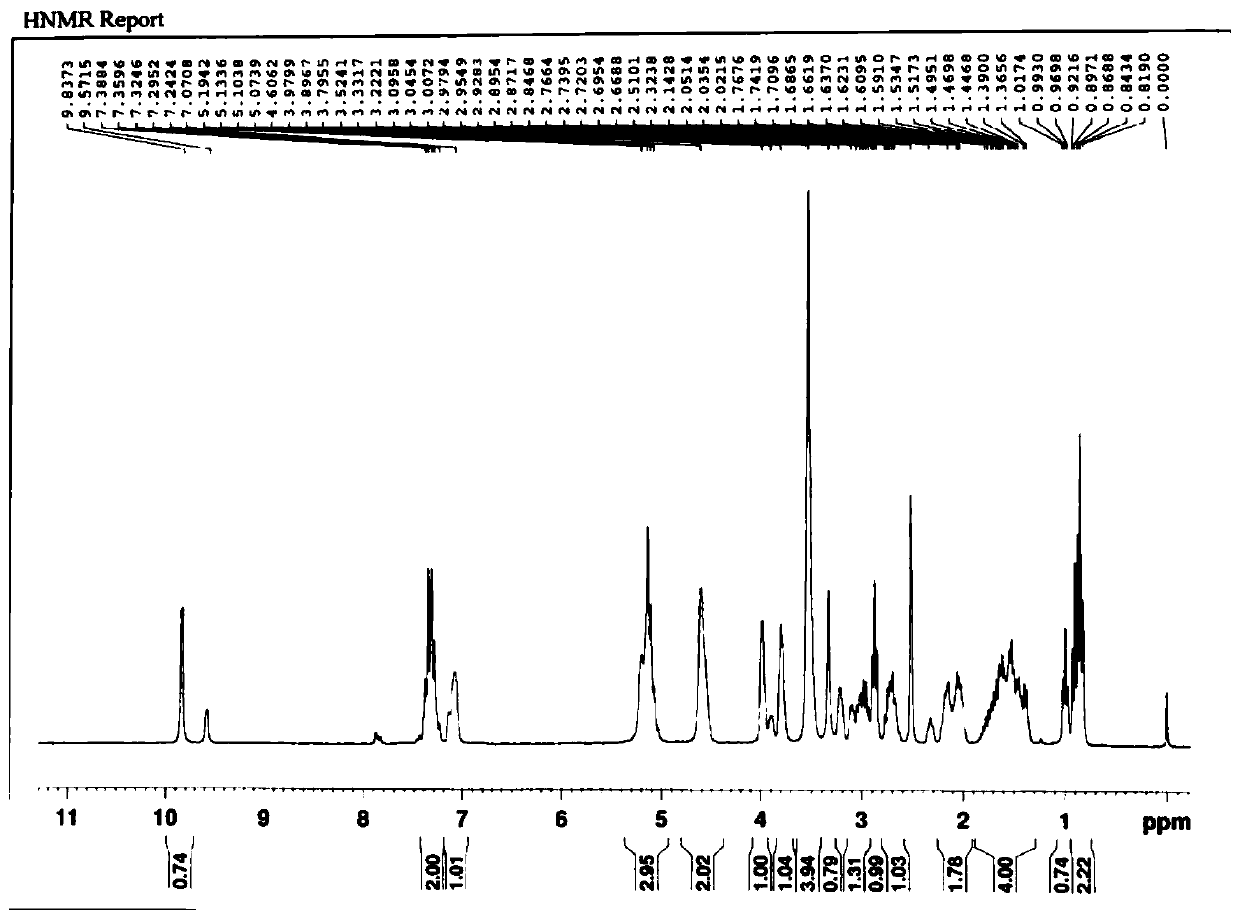
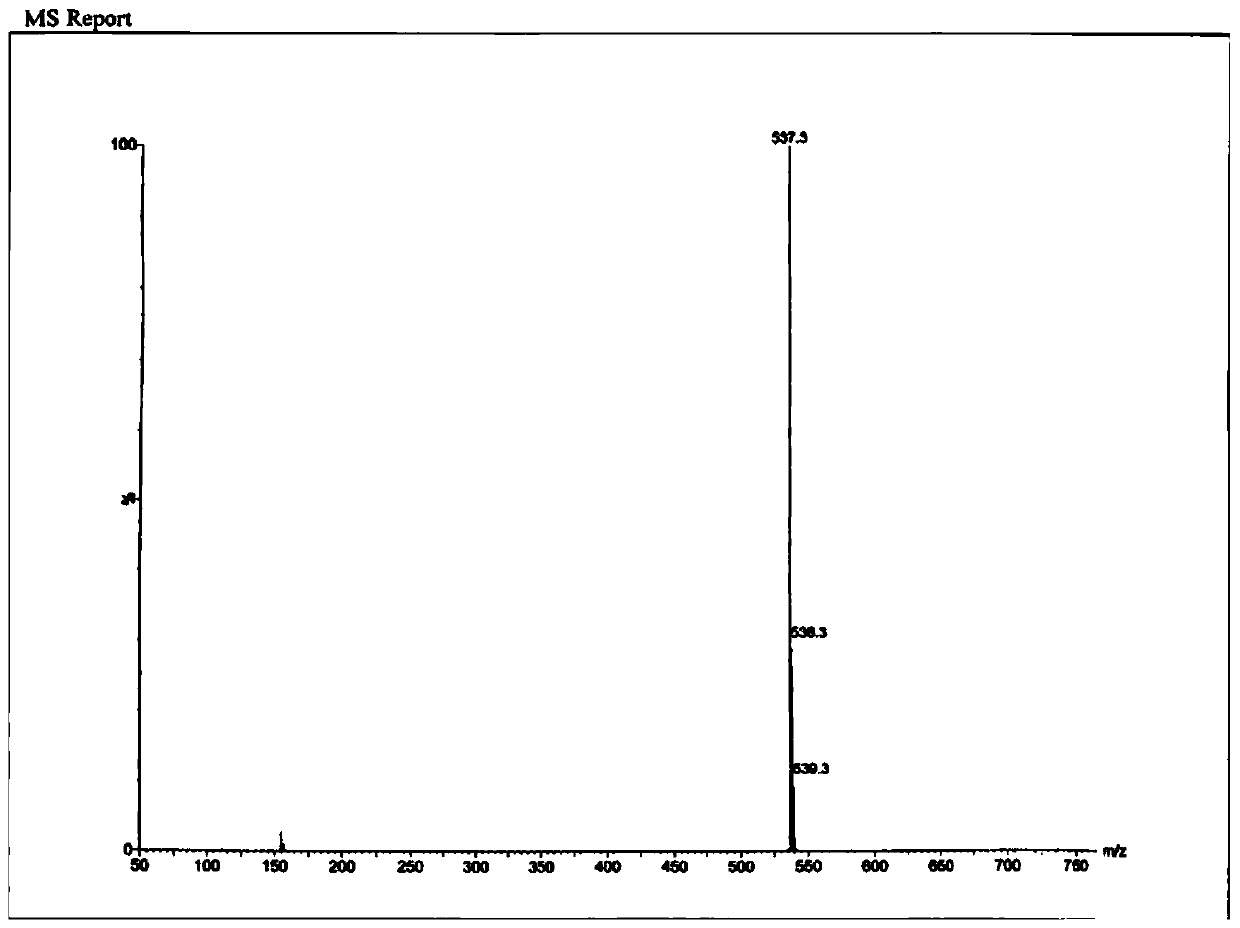
[0049] Example 1 A kind of R-type chiral sulfoxide compound
[0050] The R-type chiral sulfoxide compound was prepared and separated by the following method:
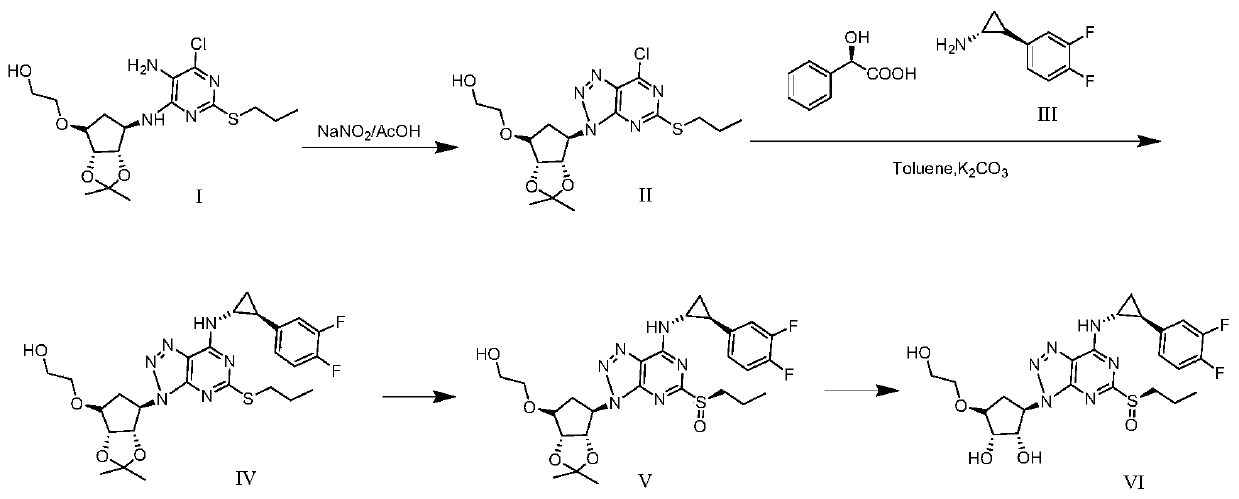
[0051](1) Dissolve 15.00g (35.80mmol) of compound I in 300mL of glacial acetic acid, add 120mL of deionized water to dilute, cool down to 4°C in an ice-salt bath, slowly add 25mL of sodium nitrite aqueous solution (containing 3.71g of sodium nitrite , 53.77mmol), heated to 30°C after dropping, reacted for 2.5h, added potassium carbonate to adjust the pH to neutral, extracted 3 times with 600mL ethyl acetate, combined organic layers, washed with saturated sodium chloride, dried and concentrated , to obtain 14.50g of compound II, yield 94.20%, purity 99.37%, ESI (m / z) [M-H] - :430.09;
[0052] (2) Dissolve 14.50g (33.73mmol) of compound II and 13.01g (1.2mmol) of compound III obtained in step (1) in 100mL of toluene, add 13.98g of potassium carbonate, stir and react at 30°C for 24h, concentrate under reduced pressure, a...
Embodiment 2
[0055] Example 2 A kind of R-type chiral sulfoxide compound
[0056] The difference from Example 1 is that the chiral reagent in Example 2 is S-mandelic acid, the oxidant is cumene hydroperoxide, and the remaining parameters and operations refer to Example 1 to obtain compound VI with a yield of 66.8% and a purity of 99.68%. %.
Embodiment 3
[0057] Example 3 A kind of R-type chiral sulfoxide compound
[0058] The difference from Example 1 is that the chiral reagent in Example 3 is (S,S)-1,2-diphenylethylene glycol, the oxidizing agent is hydrogen peroxide, and the remaining parameters and operations refer to Example 1 to obtain Compound VI, yield 67.54%, purity 99.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com