Polysubstitution resveratrol spin-labeling derivative and preparing method and application thereof
A technology of resveratrol and spin labeling, which can be used in drug combinations, pharmaceutical formulations, active ingredients of hydroxyl compounds, etc., can solve the problems of limited routes of administration, narrow time windows, limitations, etc., and achieve low cytotoxicity and repeatability. Good, the effect of reducing the level of reactive oxygen species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
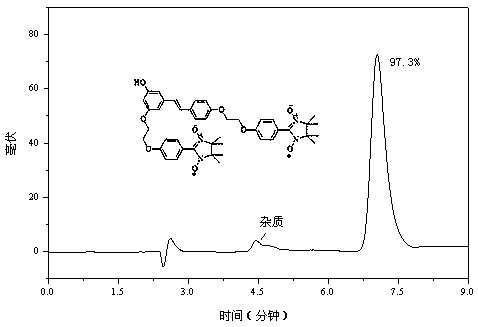
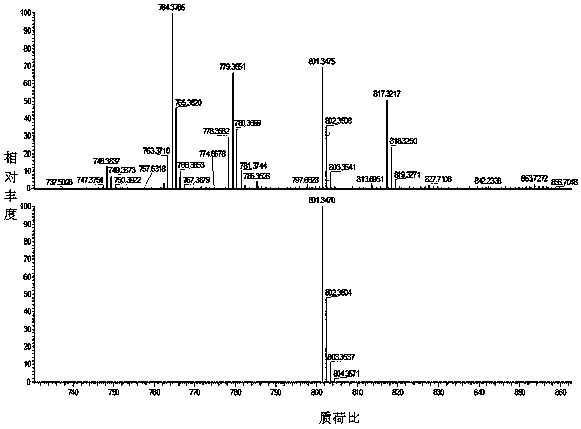
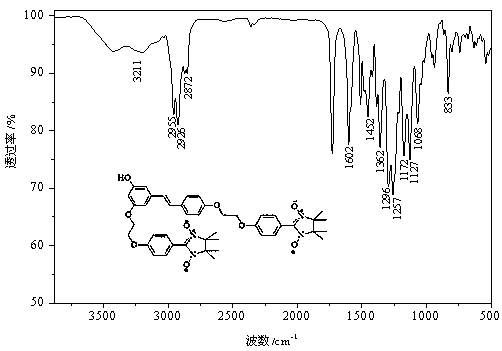
Image
Examples
Embodiment 1
[0074] Example 1 Synthesis of Disubstituted Resveratrol Nitroxide Free Radical Derivatives
[0075] Weigh 88 mg (0.38 mmol) of resveratrol, 138.2 mg (1 mmol) of anhydrous potassium carbonate and 8.3 mg of potassium iodide in a 100 mL three-necked round-bottomed flask filled with 10 mL of DMF, and slowly heat to 60 °C under nitrogen protection. Stir for 30 min, then weigh 88.8 mg (0.25 mmol ) into the reaction system, keep the temperature constant, and stir for 10 h.
[0076]At the end of the reaction, add 80 mL of distilled water to the reaction solution, then pour the resulting solution into a separatory funnel, measure 100 mL of ethyl acetate, first take a small amount of washing reaction bottle, and add the remaining ethyl acetate to the funnel for extraction. Stand still, separate the organic layer, dry the organic phase with anhydrous sodium sulfate, filter, and finally remove the solvent under reduced pressure, and purify by column chromatography (ethanol:dichloromethan...
Embodiment 2
[0092] Example 2 Synthesis of Disubstituted Resveratrol Nitroxide Free Radical Derivatives
[0093] Weigh 132 mg (0.57 mmol) of resveratrol, 207.3 mg (1.5 mmol) of anhydrous potassium carbonate and 11 mg of potassium iodide in a 100 mL three-necked round-bottomed flask filled with 15 mL of DMF, and slowly heat to 70 °C under nitrogen protection. After stirring for 30 min, add 4,4,5,5-tetramethyl-2-[4-(2-bromoethoxy)phenyl]-1,3-dioxypyrroline 133.2 mg ), TLC followed the reaction, and the reaction was completed after 15 h. Add 150 mL of distilled water to the reaction solution, pour the obtained solution into a separatory funnel, extract with ethyl acetate (80 mL×3), and use a large amount of saturated salt for the organic phase. Wash with water, finally collect the organic phase, dry over anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and purify by column chromatography (ethanol:dichloromethane=1:30~60, v / v) to obtain 60 mg of dark blue solid, chr...
Embodiment 3
[0095] Example 3 Synthesis of Disubstituted Resveratrol Nitroxide Free Radical Derivatives
[0096] Weigh 264 mg (1.14 mmol) of resveratrol, 414.6 mg (3 mmol) of anhydrous potassium carbonate and 25 mg of potassium iodide in a 100 mL three-necked round-bottomed flask filled with 15 mL of DMF, and slowly heat to 50 °C under nitrogen protection. After stirring for 30 min, 266.4 mg of 4,4,5,5-tetramethyl-2-[4-(2-bromoethoxy)phenyl]-1,3-dioxypyrroline (0. 75mmol), thin-layer chromatography followed the reaction, and the reaction was completed after 24 h. Add 150 mL of distilled water to the reaction solution, pour the obtained solution into a separatory funnel, extract with ethyl acetate (80 mL×3), and use a large amount of saturated Wash with salt water, finally collect the organic phase, dry over anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and purify by column chromatography (ethanol:dichloromethane=1:30~60, v / v) to obtain the product as a dark b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com