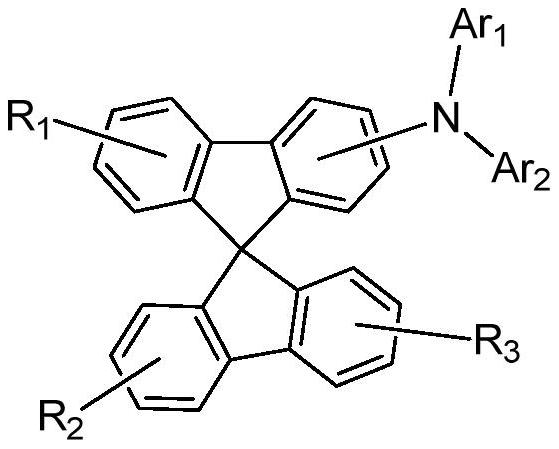
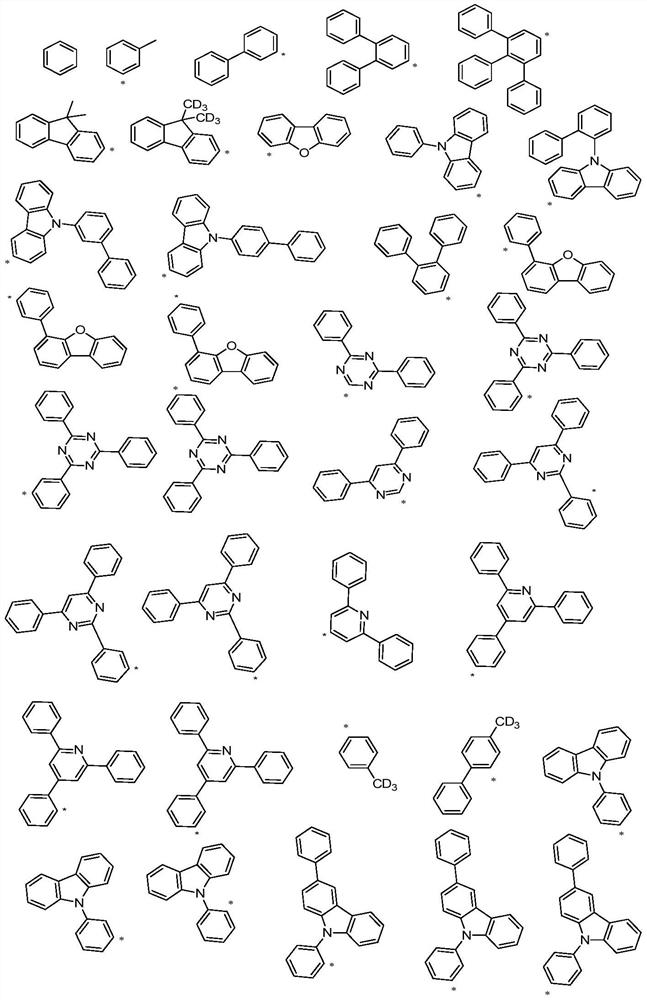
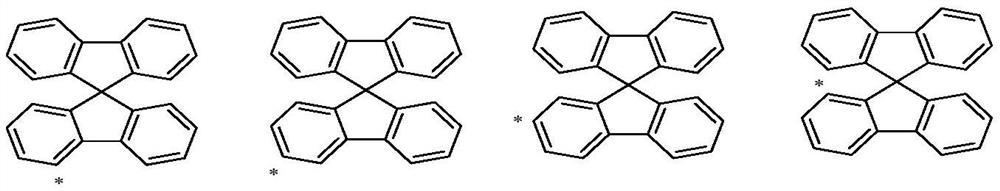
Spirobifluorene organic electroluminescent compound and organic electroluminescent device
The technology of a compound, spirobifluorene, is applied in the field of organic electroluminescent materials, which can solve the problems of insufficient development of organic electroluminescent materials and backward panel manufacturing enterprises, and achieve good aromaticity and thermal stability, reduce planarity and crystallization Sexuality and the effect of reducing manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] The specific synthetic method of compound G20 is as follows:
[0055]
[0056] (1) Compound 1 (0.1mol, 25.7g), FeCl3 (0.1eq, 0.01mol, 1.62g), CS 2 (514g, 20 times the mass of compound 1) was added to the reaction flask, chlorobutane (1.1eq, 0.11mol, 10.18g) was added under ice bath, and after the addition was completed, the room temperature was slowly returned to the reaction for 10h, and the reaction solution was poured into Ice cubes (1028g, the mass of ice cubes is CS 2 2 times of mass), hydrochloric acid was added dropwise until the pH of the system reached 2-3, and then dichloromethane (1028g, CS 2 2 times the mass of the compound) for extraction, dichloromethane phase separation, washing with water several times, drying over anhydrous sodium sulfate, and concentrating under reduced pressure to obtain the crude product of compound 2, which was purified by column chromatography to obtain the pure product of compound 2 (18.3g, harvested rate 58.3%), ...
Embodiment 2
[0063]
[0064] The specific synthetic method of compound G116 is as follows:
[0065]
[0066] (1) Compound 7 (1eq, 288g / mol, 57mmol, 16.4g) and anhydrous THF (164g, 10 times the mass of compound 7) were added to the reaction flask under the protection of nitrogen. The liquid nitrogen was cooled to -78°C, and added dropwise n-Butyllithium (1.1eq, 62.7mmol), after reacting for 30min, compound 1 (14.7g, 257.97g / mol, 57mmol), anhydrous THF (147g, 10 times the mass of compound 2) were mixed and added dropwise, Continue to react at -78°C for 2 hours, then add ammonium chloride solution to quench, slowly return to room temperature, add dichloromethane and water for extraction and separation, separate the dichloromethane phase, wash with water several times, dry over anhydrous sodium sulfate, reduce Concentrate under reduced pressure to obtain the crude product of compound 8, obtain the pure product of compound 8 (15.2g, yield 57.1%) after column chromatography purification, M...
Embodiment 3
[0072]
[0073] The specific synthetic method of compound G148 is as follows:
[0074] Operation in step (1) and step (2) is basically the same as in Example 2, and step (3) is as follows:
[0075]
[0076] (3) Under nitrogen protection, compound 9 (10g, 22.2mmol), compound 10 (1.1eq, 321g / mol, 24.4mmol, 7.84g), sodium tert-butoxide (1.1eq, 96.1g / mol, 24.4mmol, 2.35g), Pd 2 (dba) 3 (5%eq, 915.72g / mol, 1.11mmol, 1.02g), tri-tert-butylphosphine (5%eq, 202.317g / mol, 1.11mmol, 0.223g), toluene (100g, 10 times the mass of compound 9) Add it into the reaction flask, heat up to reflux reaction for 5 hours after the addition, cool down to room temperature after the reaction, add water and stir for 15 minutes, and then filter to obtain the filtrate. After the filtrate is separated, the organic phase is obtained. After purification by column chromatography, high-purity G148 (7.63g, yield 49.9%) was obtained, MS (EI): 691 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com