A kind of method for separating and measuring Ribociclib and impurities
A ribociclib and impurity technology, applied in the field of analytical chemistry, can solve the problems of difficult to achieve effective separation of ribociclib, unfavorable to the quality control of ribociclib, increase the difficulty of detection, etc., and achieve accurate and reliable detection results. , eliminate solvent effect, improve the effect of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
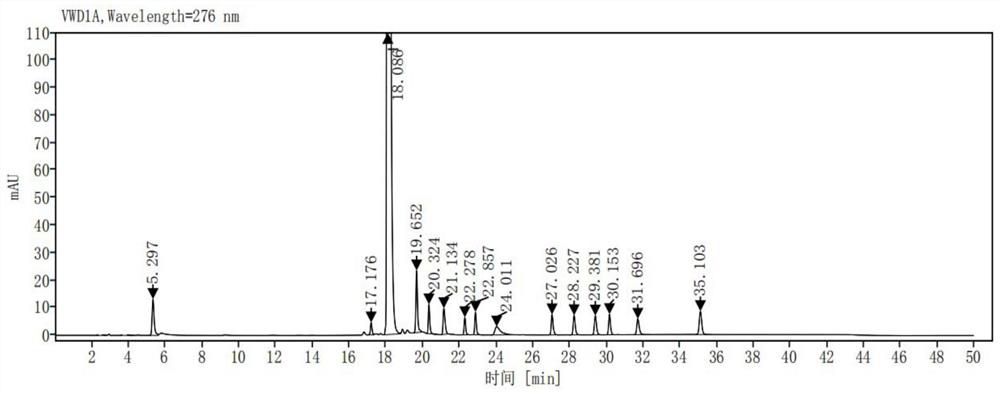
[0034] Take impurity Z1, impurity Z2, impurity Z3, impurity Z4, impurity Z5, impurity Z6, impurity Z7, impurity Z8, impurity Z9, impurity Z10, impurity Z11, impurity Z12, impurity Z13, impurity Z14 (the purity of each impurity is in More than 99%, each impurity is provided by Chongqing Sansheng Industrial Co., Ltd.) 25mg each, accurately weighed, placed in a 100ml measuring bottle, add acetonitrile-0.01mol / L potassium dihydrogen phosphate solution (pH is 3.0) (volume The ratio is 10:90) dissolved and diluted to the scale, shaken up, as the impurity stock solution; take about 25 mg of ribociclib (provided by Chongqing Sansheng Industrial Co., Ltd., the purity is 99.88%), accurately weighed, placed in In a 50ml volumetric flask, accurately add 1ml of the impurity stock solution, add acetonitrile-0.01mol / L potassium dihydrogen phosphate solution (pH is 3.0) (volume ratio is 10:90) to dissolve and dilute to the mark, shake well, as a mixed control solution .
[0035] Take the dil...
Embodiment 2
[0039] Example 2 Determination of ribociclib API (provided by Chongqing Sansheng Industrial Co., Ltd.)
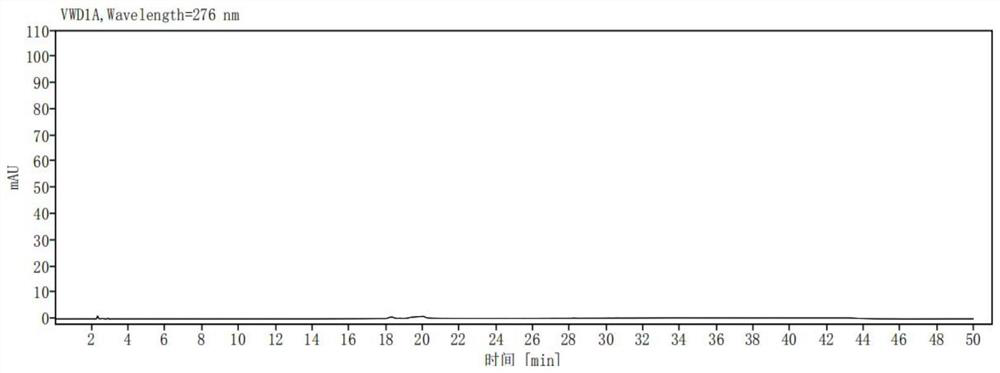
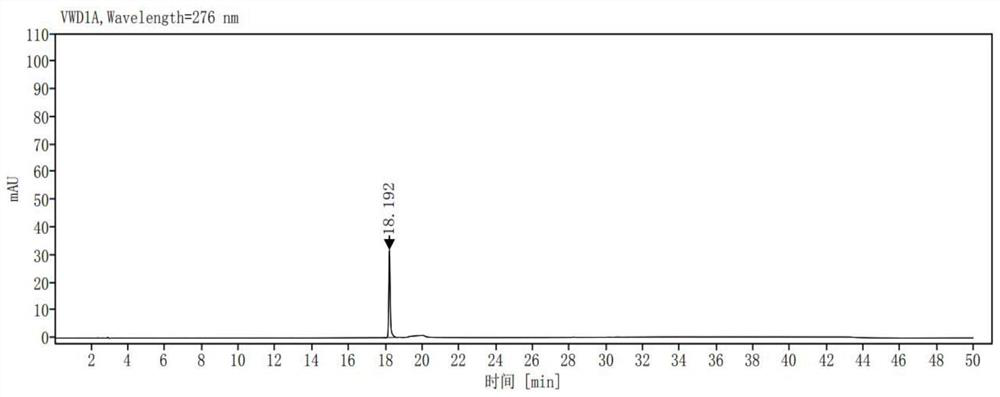
[0040] Take ribociclib 25mg, accurately weigh it, put it in a 50ml measuring bottle, add (acetonitrile-0.01mol / L potassium dihydrogen phosphate solution (pH is 3.0)=10:90) ultrasonic treatment to dissolve and dilute to the mark, shake Evenly, as a sample solution; accurately measure 1ml of the sample solution, place it in a 100ml measuring bottle, dilute it to the mark with (acetonitrile-0.01mol / L potassium dihydrogen phosphate solution (pH 3.0)=10:90), shake well , as the control solution; carry out liquid chromatographic analysis according to the chromatographic conditions of Example 1, and record the chromatogram. If there are impurity peaks (except solvent peaks) in the chromatogram of the sample solution, calculate the impurity content according to the self-control method. The result is as image 3 , Figure 4 shown. The test results are shown in Table 1:
[0041]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com