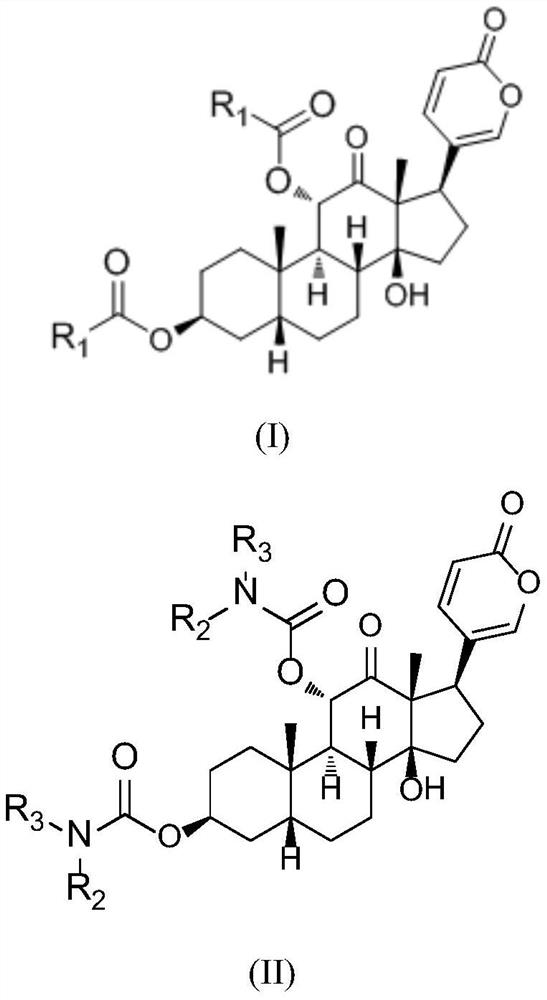
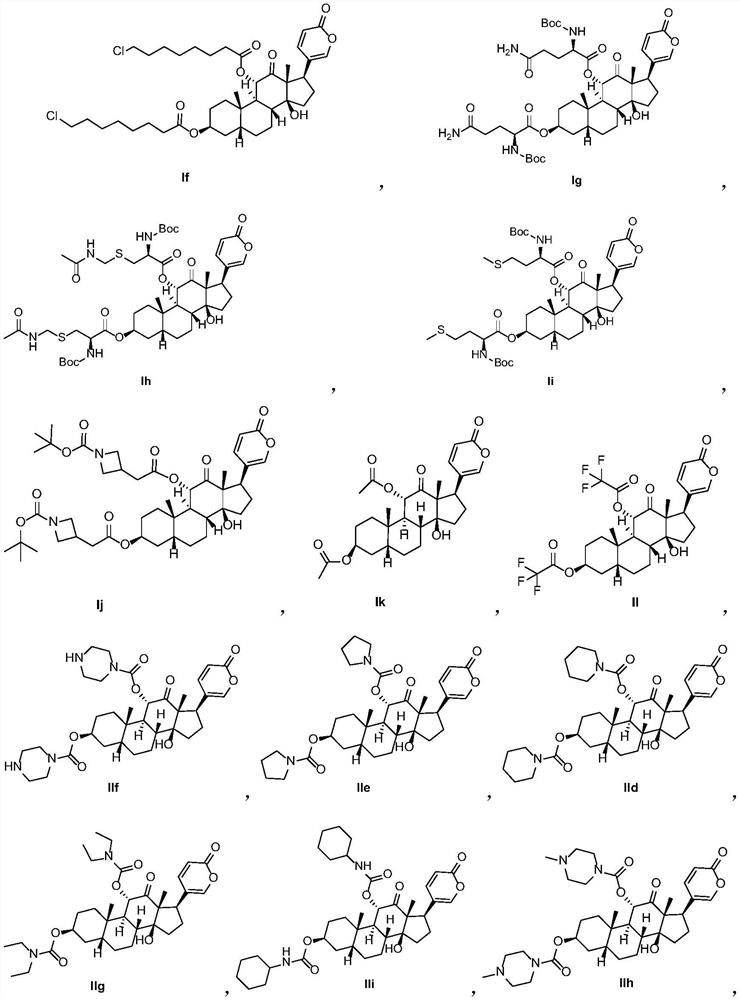
Arenobufagin derivative, preparation method thereof, pharmaceutical composition of arenobufagin derivative and application ofarenobufagin derivative
A technology of sandbuad toxin and its derivatives, which is applied in the field of medicine and can solve the problems of narrow therapeutic index and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
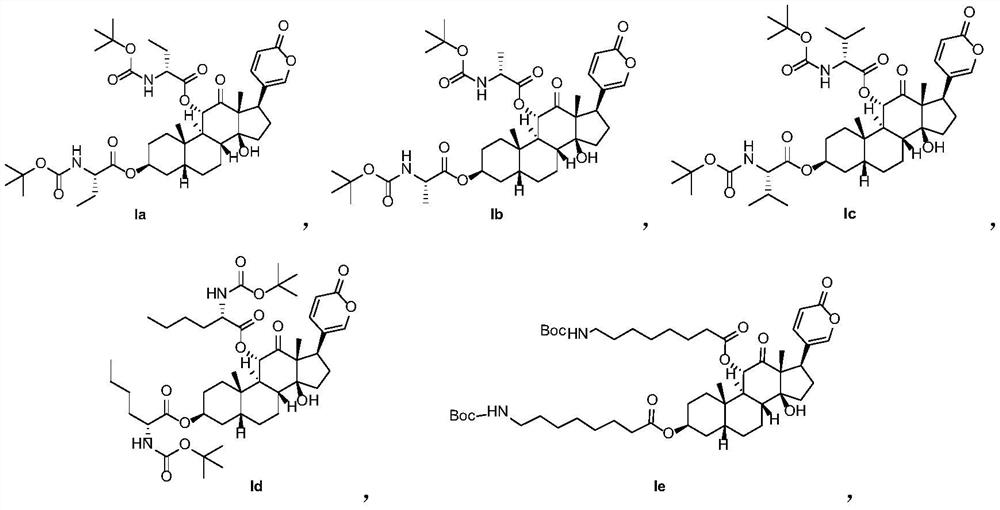
[0081] The preparation of embodiment 1 compound Ia
[0082]
[0083] Add sabubaptin (43mg, 0.1mmol), N-Boc-D-aminobutyric acid (101.6mg, 0.5mmol) and DMAP (48.8mg, 0.2mmol) into a 25mL flask, add 5mL di Chloromethane. Then EDCI (230mg, 1.2mmol) was added in batches, stirred overnight at room temperature, the solvent was evaporated, and 36.4mg of white solid was obtained by column chromatography with a yield of 46.3%. 1 H NMR (500MHz, CDCl 3 )δ:7.74(d,1H),7.35(s,1H),6.23(d,1H),5.39(s,1H),5.00–5.15(m,3H),3.99–4.36(m,3H),2.91 –3.02(m,1H),1.23–2.18(m,39H),0.83–1.10(m,12H).ESI-HRMS calcd C42H62N2O12[M+Na] + m / z, 809.4200; found 809.4206.
Embodiment 2
[0084] The preparation of embodiment 2 compound 1b
[0085]
[0086] Referring to the preparation method of compound Ia in Example 1, N-Boc-D-aminobutyric acid was replaced by N-Boc-D-aminobutyric acid to obtain a white solid with a yield of 44.3%. 1 H NMR (500MHz, CDCl 3 )δ:7.72(d,1H),7.38(s,1H),6.28(d,1H),5.43(d,1H),5.00–5.16(m,3H),4.03–4.32(m,3H),1.72 –2.28(m,12H),1.26–1.57(m,28H),1.15(s,3H),0.99(s,3H).ESI-HRMS calcd C40H58N2O12[M+Na] + m / z, 781.3887; found 781.3886.
Embodiment 3
[0087] The preparation of embodiment 3 compound Ic
[0088]
[0089] Referring to the preparation method of compound Ia in Example 1, N-Boc-D-aminobutyric acid was replaced by N-Boc-D-valine to obtain a white solid with a yield of 39.6%. 1 H NMR (500MHz, CDCl 3 )δ: 7.74(d,1H),7.38(s,1H),6.26(d,1H),5.38(d,1H),5.15(s,1H),4.96–5.05(m,2H),4.03–4.19 (m,3H),1.76–2.23(m,14H),1.26–1.51(m,24H),1.12(s,3H),1.03–1.07(m,6H),0.97–0.99(m,6H),0.89 (d,3H).ESI-HRMS calcd C44H66N2O12[M+Na] + m / z, 837.4513; found 837.4511.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com