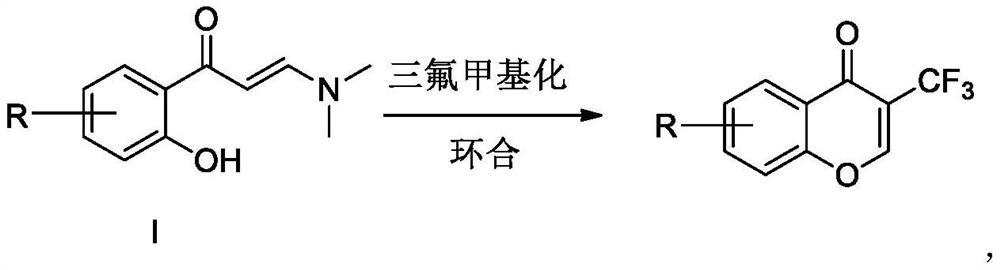
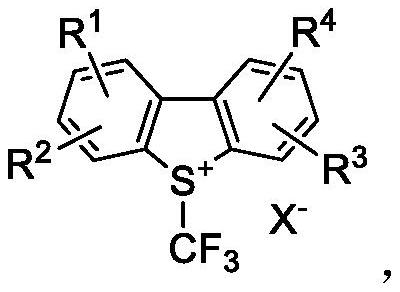
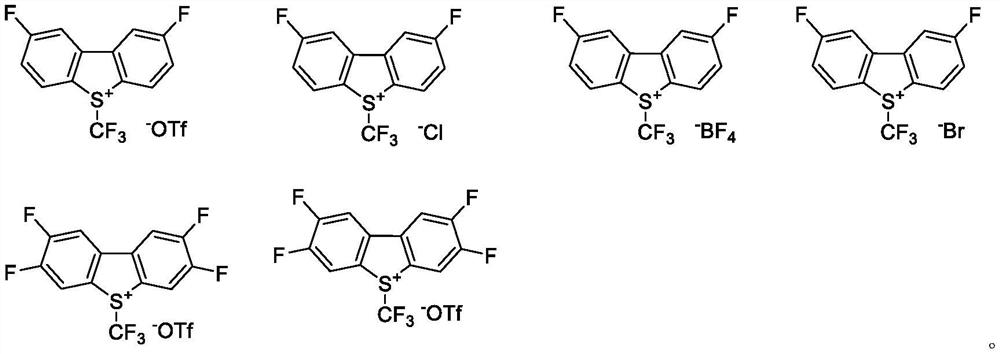
Preparation method of trifluoromethyl chromone compound
A technology of trifluoromethyl chromone and trifluoromethylation, which is applied in the field of preparation of trifluoromethyl chromone compounds, can solve the problems of unsuitability for industrialization and high cost, and achieve creative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Under nitrogen protection, add 1.0mmol 1a, 1.2mmol Umeben's reagent 2a, 5mL DMF, and 1.5mmol DMAP into a 10mL reaction tube. Placed in an oil bath at 80°C for 3h, then moved to room temperature. Add 20 mL of water, extract with ethyl acetate three times (25 mL×3), combine the ethyl acetate layers, wash with water three times (20 mL×3), and dry over anhydrous magnesium sulfate. Suction filtration, concentration to dryness under reduced pressure, and purification by column chromatography gave the target product with a yield of 75%.
Embodiment 2
[0025]
[0026] Under nitrogen protection, add 1.0mmol 1a, 1.2mmol Umeben's reagent 2a, 5mL CH 3 CN, 1.5 mmol DMAP. Placed in an oil bath at 80°C for 3h, then moved to room temperature. Add 20 mL of water, extract with ethyl acetate three times (25 mL×3), combine the ethyl acetate layers, wash with water three times (20 mL×3), and dry over anhydrous magnesium sulfate. Suction filtration, concentration to dryness under reduced pressure, and purification by column chromatography gave the target product with a yield of 65%.
Embodiment 3
[0028]
[0029] Under nitrogen protection, add 1.0mmol 1a, 1.2mmol Umeben's reagent 2a, 5mL DMSO, 1.5mmol DMAP into a 10mL reaction tube. Placed in an oil bath at 80°C for 3h, then moved to room temperature. Add 20 mL of water, extract with ethyl acetate three times (25 mL×3), combine the ethyl acetate layers, wash with water three times (20 mL×3), and dry over anhydrous magnesium sulfate. Suction filtration, concentration to dryness under reduced pressure, and purification by column chromatography gave the target product with a yield of 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com