Universal CpGODN nanoparticle adjuvant as well as preparation method and application thereof
A nanoparticle and adjuvant technology, which is applied in the field of universal CpGODN nanoparticle adjuvant and its preparation, can solve the problems of increasing CpGODN immune effect, CpGODN toxicity and side effects, limiting the use range of CpGODN immune adjuvant, body toxicity and side effects, etc., to achieve Good immune effect, improved utilization and safety, and reduced systemic inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
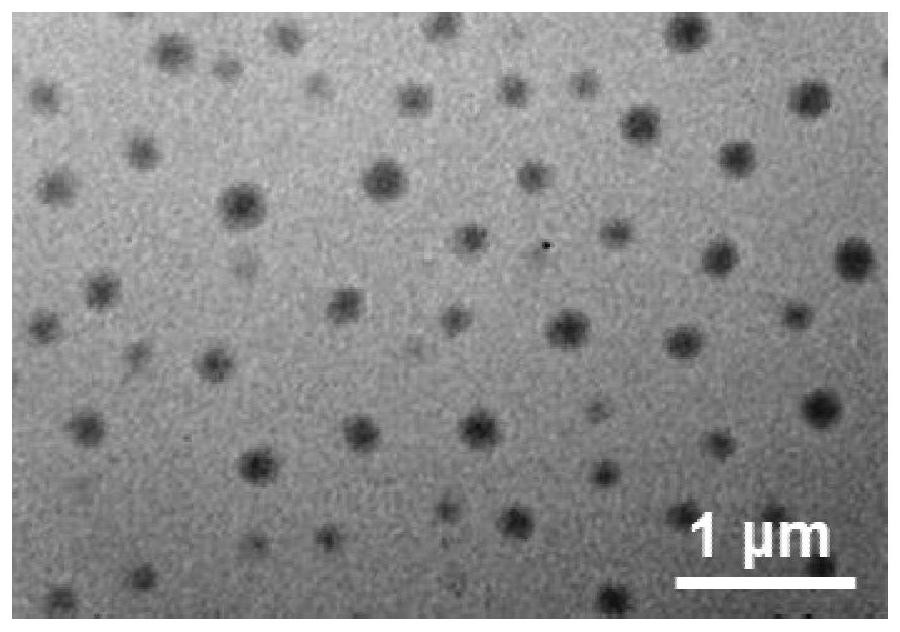
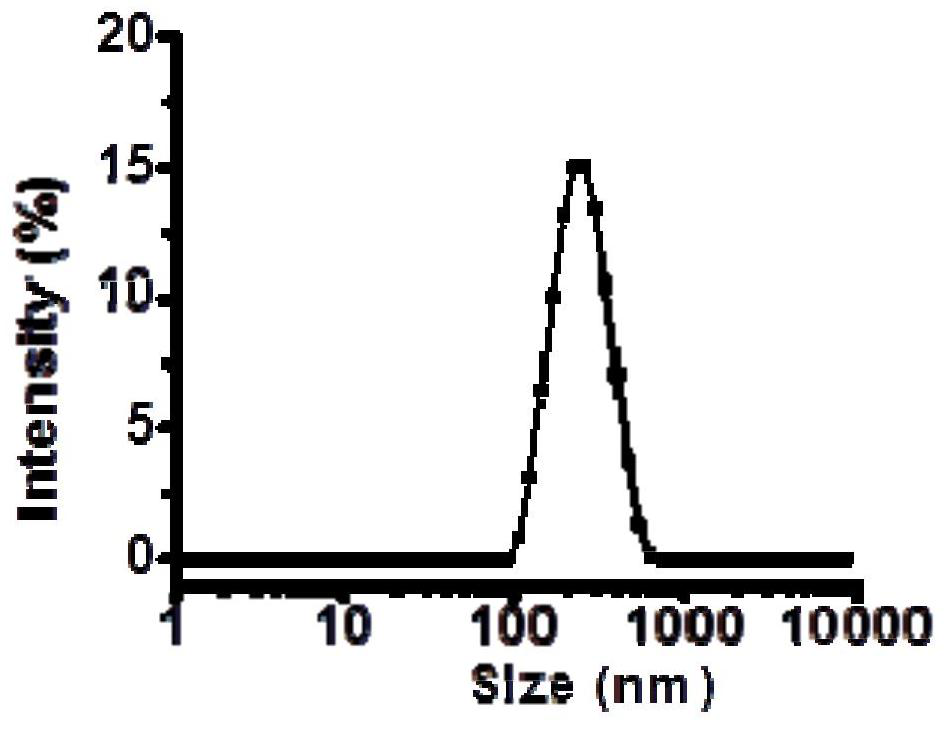
Image
Examples
Embodiment 1
[0047] Example 1 Preparation method of nanoparticle adjuvant comprising CpG ODN
[0048] 1. Method
[0049] 1. Solution preparation
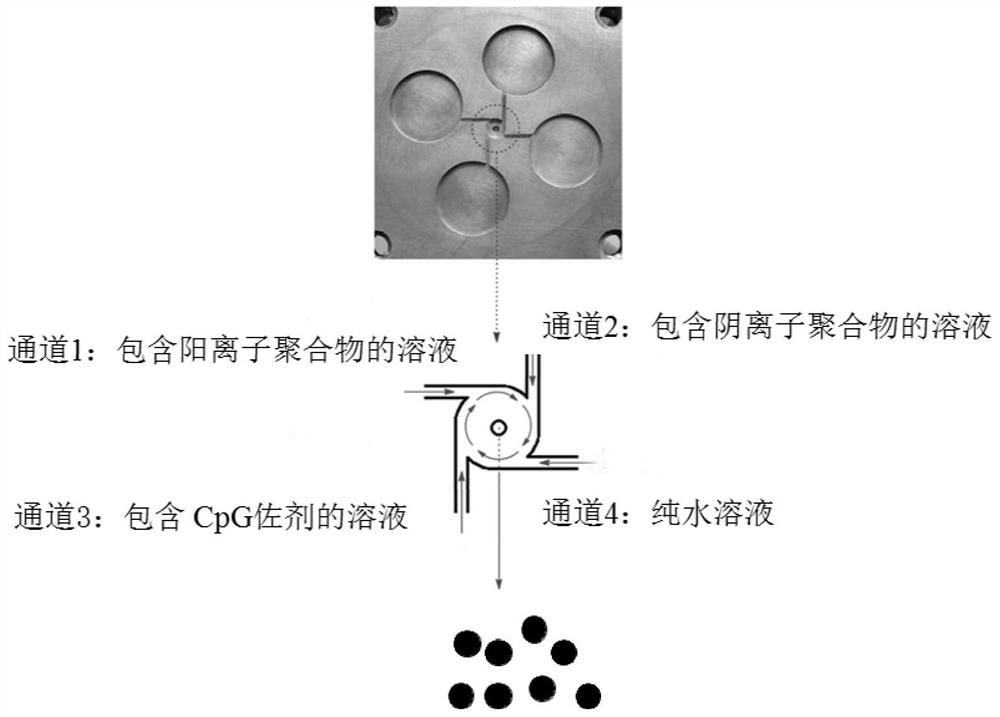
[0050] (1) Disperse protamine in sterilized distilled water, stir overnight under magnetic stirring, and filter through filter paper to obtain a protamine solution. (2) Disperse sodium tripolyphosphate in sterilized distilled water, stir for 5 minutes under magnetic stirring, and filter through a 0.22 μm filter membrane to obtain sodium tripolyphosphate solution. (3) Dissolve CpG in distilled water, stir for 5 minutes under magnetic stirring, and filter through a 0.22 μm filter membrane to obtain a CpG solution. (4) Using the method of fast nanocomplexation (FNC) (such as figure 1 ), channel 1: protamine solution; channel 2: sodium tripolyphosphate solution; channel 3: CpG-containing solution; channel 4: pure water, the flow rate of the syringe pump is 10 mL / min, and solution A can be obtained. (5) The concentration of protamine in step (1) ...
Embodiment 2
[0061] Example 2 Preparation method of nanoparticle adjuvant comprising CpG ODN
[0062] 1. Method
[0063] 1. Solution preparation
[0064] (1) Disperse protamine in sterilized distilled water, stir overnight under magnetic stirring, and filter through filter paper to obtain a protamine solution. (2) Disperse sodium tripolyphosphate in sterilized distilled water, stir for 5 minutes under magnetic stirring, and filter through a 0.22 μm filter membrane to obtain sodium tripolyphosphate solution. (3) Dissolve CpG in distilled water, stir for 5 minutes under magnetic stirring, and filter through a 0.22 μm filter membrane to obtain a CpG solution. (4) Using the method of fast nanocomplexation (FNC) (such as figure 1 ), channel 1: protamine solution; channel 2: sodium tripolyphosphate solution; channel 3: CpG-containing solution; channel 4: pure water, and the flow rate of the syringe pump was 10 mL / min to obtain solution B. (5) The concentration of protamine in step (1) is 0.5...
Embodiment 3
[0075] Example 3 Preparation method of nanoparticle adjuvant comprising CpG ODN
[0076] 1. Method
[0077] 1. Solution preparation
[0078] (1) Disperse protamine in sterilized distilled water, stir overnight under magnetic stirring, and filter through filter paper to obtain a protamine solution. (2) Disperse sodium tripolyphosphate in sterilized distilled water, stir for 5 minutes under magnetic stirring, and filter through a 0.22 μm filter membrane to obtain sodium tripolyphosphate solution. (3) Dissolve CpG in distilled water, stir for 5 minutes under magnetic stirring, and filter through a 0.22 μm filter membrane to obtain a CpG solution. 4. Using fast nanocomplexation (FNC) methods (such as figure 1 ), channel 1: protamine solution; channel 2: sodium tripolyphosphate solution; channel 3: CpG-containing solution; channel 4: pure water, the flow rate of the syringe pump is 10 mL / min, and solution C can be obtained. (5) The concentration of protamine in step (1) is 0.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com