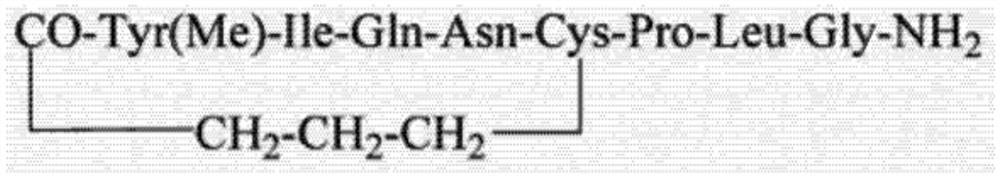Synthesis method of carbetocin
A technology for carbetocin and a synthesis method, applied in the field of polypeptide drug preparation, can solve the problems of poor continuity, unfavorable purification process development, low overall process yield and the like, and achieves reduction of comprehensive production cost, improvement of cyclization reaction efficiency, The effect of process green environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
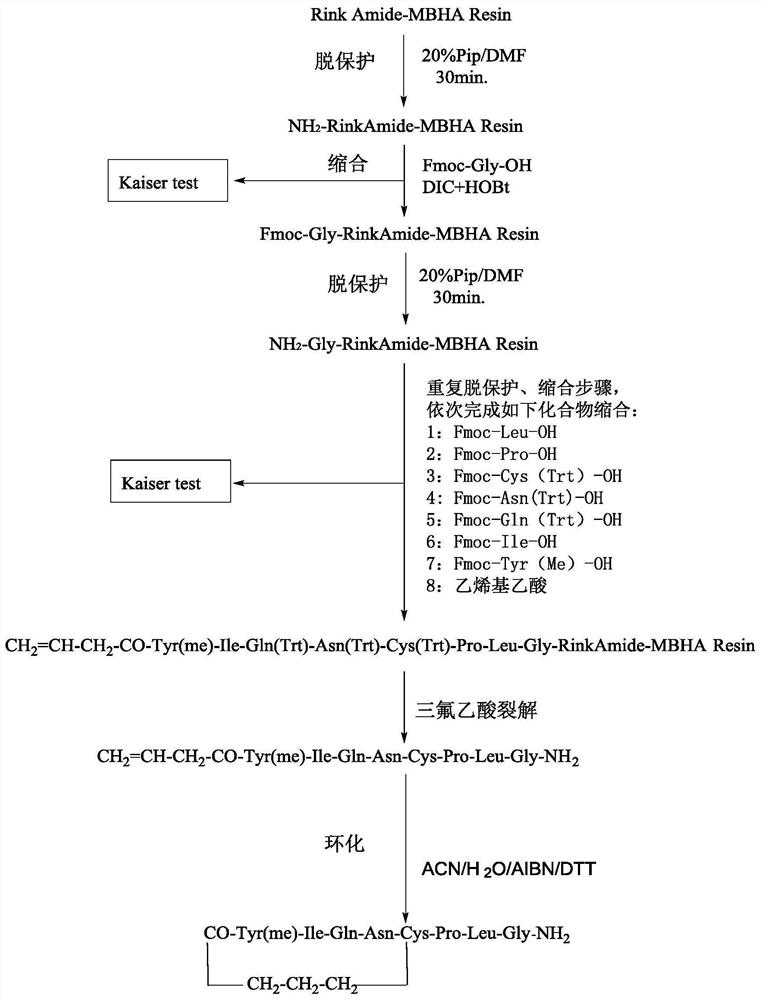
[0050] The synthetic method of carbetocin in some examples of the present invention comprises the following steps:
[0051] (1) With Rink Amide-MBHA Resin as the carrier, after swelling treatment, the protected Gly, protected Leu, protected Pro, protected Cys, protected Asn, protected Gln, protected Ile and protected Tyr were condensed in sequence The reaction is coupled to the swelling Rink Amide-MBHA Resin, and dried to obtain the peptide resin 1;
[0052] (2) Peptide resin 1 reacts with vinyl acetic acid to obtain peptide resin 2, which has the following structure: CH 2 =CH-CH 2 -CO-Tyr(me)-Ile-Gln(Trt)-Asn(Trt)-Cys(Trt)-Pro-Leu-Gly-Rink Amide-MBHA Resin;
[0053] (3) Peptide resin 2 is cracked to obtain carbetocin intermediate I, whose structure is as follows:
[0054] CH 2 =CH-CH 2 -CO-Tyr(me)-Ile-Gln(Trt)-Asn(Trt)-Cys(Trt)-Pro-Leu-Gly-NH 2 ;
[0055] (4) Carbetocin intermediate I was cyclized to obtain carbetocin.
[0056] During the synthesis process, the coupli...
Embodiment 1
[0080] The carbetocin synthesis process of the present embodiment 1 is as follows: figure 1 As shown, specifically:
[0081] (1) Rink Amide-MBHA Resin swelling
[0082] Calculate and weigh 100mmol Rink Amide-MBHA Resin (the degree of substitution is 0.8mmol / g), pour it into the reaction kettle, add DCM in an amount of 20ml / g, and let it stand for swelling for 3 hours.
[0083] Washing: Wash twice with DMF, each time 1.0L, and each washing time is not less than 2min.
[0084] Deprotection: Add 1.0L of 20v / v% piperidine / DMF, stir for 30 minutes, then drain the reaction solution, and the reaction is terminated.
[0085] Washing: Wash twice with DMF, twice with methanol, twice more with DMF, each time with 1.0L, and each time of washing should not be less than 2min.
[0086] (2) Coupling of Fmoc-Gly-OH
[0087] Weigh Fmoc-Gly–OH: 89.25g, HOBt: 40.54g, DIC: 47.33ml.
[0088]Dissolve Fmoc-Gly-OH and HOBt with 300ml DMF, slowly add DIC to the swollen Rink Amide-MBHA Resin for pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com