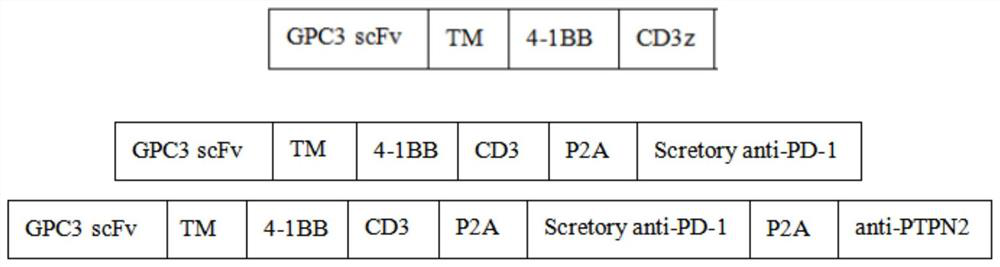
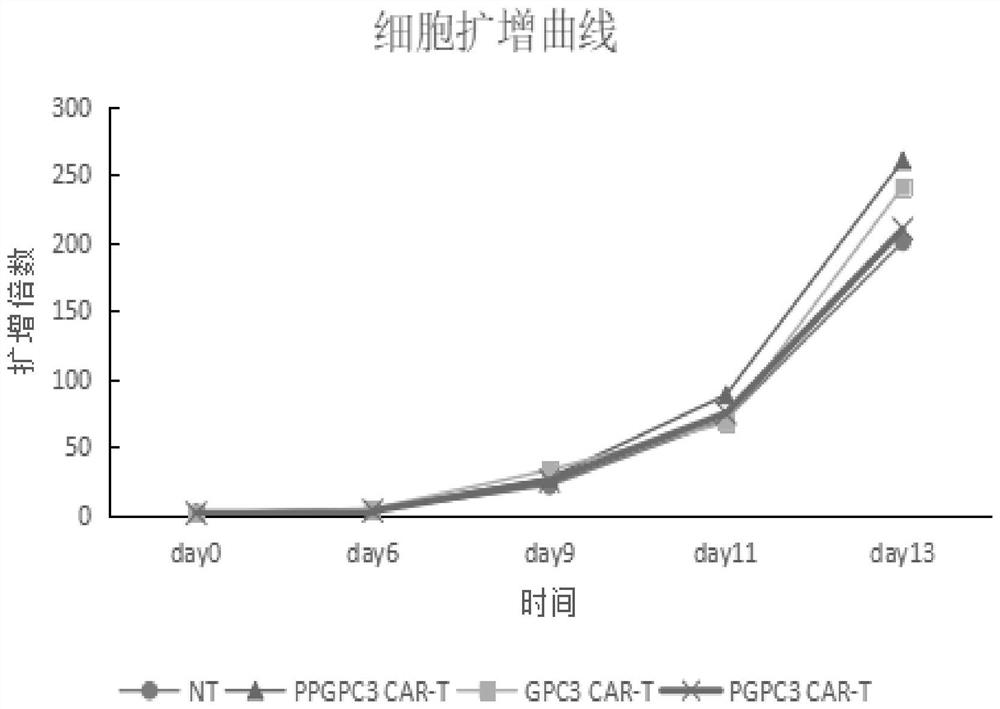
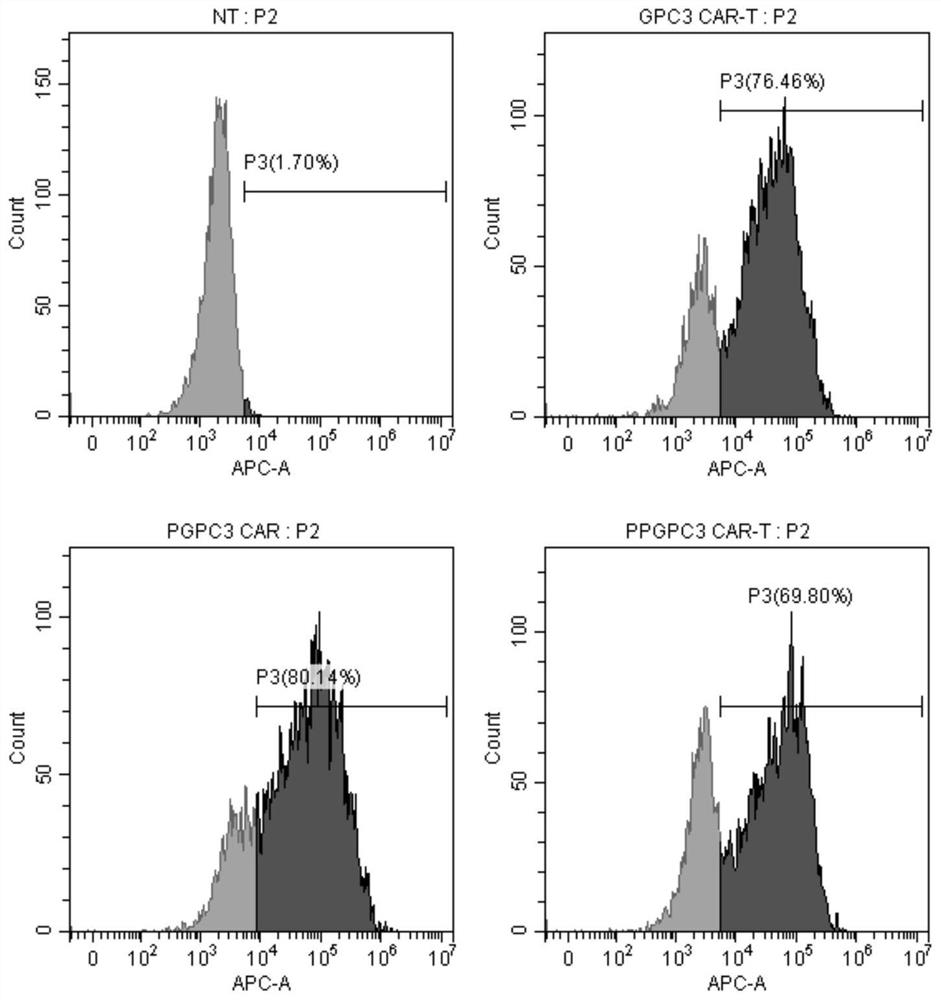
PTPN2-blocked immune cell and application and preparation thereof
A technology of immune cells and NKT cells, applied in the field of PTPN2-blocking immune cells and their applications and preparations, can solve the problems of solid tumors without good treatment effect, low clinical response rate, off-target, etc., to enhance the killing function and reduce exhaustion. , to avoid toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation method and functional verification of immune cells in this embodiment include the following steps:
[0081] Step 1: Isolation of PBMC from peripheral blood and expansion of T cells
[0082] Mononuclear cells were isolated from donor peripheral blood, density gradient centrifugation was performed using ficol, and T cells were enriched with a T cell sorting kit (CD3 MicroBeads, human-lyophilized, 130-097-043), using conjugated anti-CD3 / anti-CD28 magnetic beads activated culture and expansion of T cells; the medium used TexMACS GMP Medium (MiltenyiBiotec, 170-076-309), containing 10% FBS, 2mM L-glutamine, 100IU / ml rhIL2, all cells were placed Cultured in a constant temperature incubator at 37°C with 5% CO2.
[0083] Step 2: Cell Line Culture
[0084] Cell line expressing GPC3: Huh-7 (human liver cancer cell), purchased from ATCC.
[0085] Cell line not expressing GPC3: A549 (human non-small cell lung cancer cells), purchased from ATCC.
[0086] Cells f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com