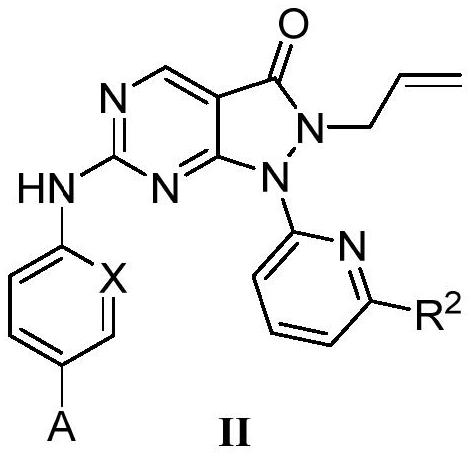
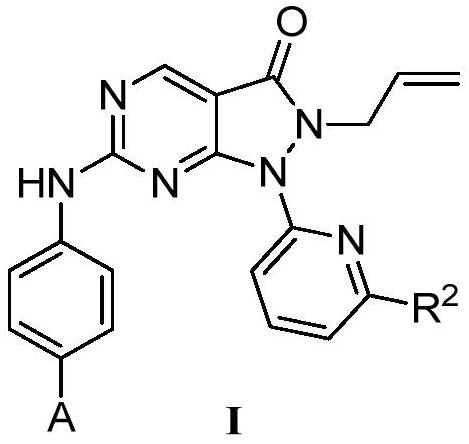
Pyrazolone-fused pyrimidine compound as well as preparation method and application thereof
A pyrazolone and pyrimidine technology, applied in the field of pyrazolone pyrimidine compounds, can solve problems such as single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0694]
[0695]first step:
[0696]4-Bromoaniline (I-1-a) (58.1mmol) was dissolved in toluene (250mL), potassium carbonate (87.2mmol) and benzyl chloride (87.2mmol) were added to the reaction solution, and the reaction solution was kept at room temperature. Stir for 16 hours. The reaction solution was filtered, and the filtrate was evaporated to dryness. The crude product was washed with ethyl acetate to obtain the target compound benzyl (4-bromophenyl) carbamate (I-1-b) (15.2 g, 85.4%) as a gray solid . LC-MS:m / z:(M+H)+= 307.0.
[0697]Step 2:
[0698]The benzyl (4-bromophenyl) carbamate (16.0 mmol) (I-1-b) was dissolved in 1,2-dimethoxyethane (50 mL), and 4,4, 5,5-Tetramethyl-2-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)-1,3,2-dioxaborane (as in formula I-1 -c) (16.0mmol), sodium carbonate (42.0mmol) and tetrakistriphenylphosphine (1.6mmol), the reaction solution was heated to 80°C and stirred for 16 hours. The reaction solution was filtered, and the filtrate was evaporated to dryness to obtain a crud...
Embodiment 3
[0708]
[0709]Compound (I-3-1) and compound (I-3-2) can be synthesized by using cyclopentane as a raw material in the same manner as in Example 1.
[0710]details as follows:
[0711]
[0712]first step:
[0713]Add tert-butyl carbamate (4'-oxo-2', 3', 4', 5'-tetrahydro-[1,1'-biphenyl]-4-yl) carbamate (0.46g, 1.6mmol) (I-8-c) and tetrahydropyrrole (0.28g, 3.9mmol) were added sodium acetate borohydride (0.85g, 4mmol) in a solution of 20ml of dichloromethane, and stirred overnight at room temperature. The reaction solution was saturated with Sodium carbonate aqueous solution (20ml), water (2*10ml) and saturated brine are washed, the organic phase is dried with anhydrous sodium sulfate and then mixed with silica gel and passed through the column {7M ammonia methanol: (dichloromethane: ethyl acetate = 12 : 2)=0-15%}, to obtain compound I-3-a, 200 mg of white solid. The yield was 40%. LC-MS: m / z: (M+H)+=343.
[0714]The second step:
[0715]Add (4'-(pyrrolidin-1-yl)-2', 3', 4', 5'-tetrahydro-[1,1'-biphenyl]...
Embodiment 4
[0723]
[0724]first step:
[0725]The 1-bromo-4-nitrobenzene) (I-4-a) (692mg, 3.43mmol), 4-(4,4,5,5-tetramethyl-1,3,2-dioxaboron Cyclopentane-2-yl) ethyl cyclohex-3-ene-1-carboxylate (I-4-b) (800mg, 2.85mmol), tetrakistriphenylphosphonium palladium (330mg, 0.286mmol), three Phenylphosphine (75mg, 0.286mmol) and potassium carbonate (789mg, 5.71mmol) were dissolved in 1,4-dioxane (20ml), heated to 90°C under argon protection and stirred for about 16 hours. Then the reaction solution was concentrated and purified by column chromatography (silica gel, petroleum ether / ethyl acetate=100% to 90%) to obtain 450 mg of the compound represented by formula I-4-c as a white solid. Yield: 47%.1H NMR(400MHz, CDCl3)δ8.23–8.16(m,2H), 7.57–7.49(m,2H), 6.33(dd,J=5.1,2.8Hz,1H), 4.26–4.15(m,2H), 2.72–2.61(m, 1H), 2.59–2.51 (m, 4H), 2.24 (ddd, J = 9.3, 8.0, 3.9 Hz, 1H), 1.90 (dddd, J = 13.1, 11.0, 8.8, 6.7 Hz, 1H), 1.31 (dd, J = 9.2, 5.1 Hz, 3H).
[0726]Step 2:
[0727]Dissolve 4-(4-nitrophenyl)cyclohex-3-ene-1-ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com