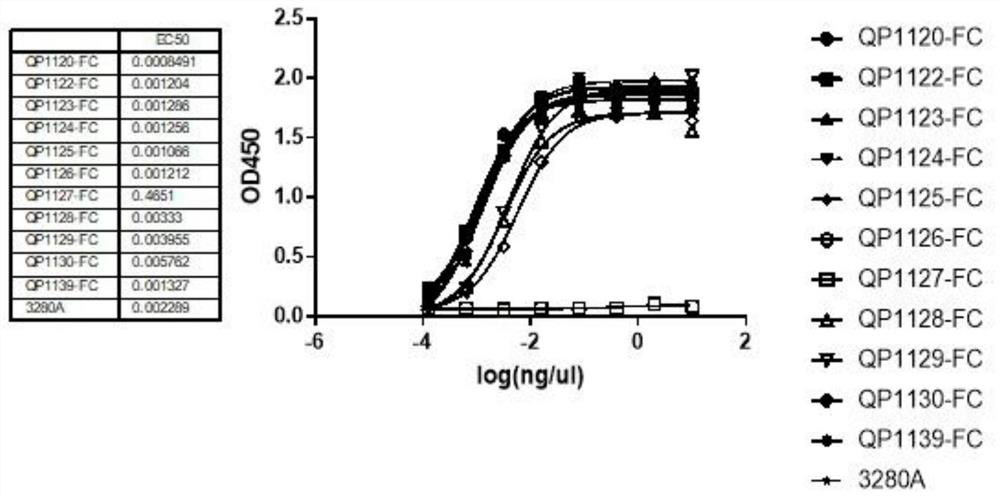
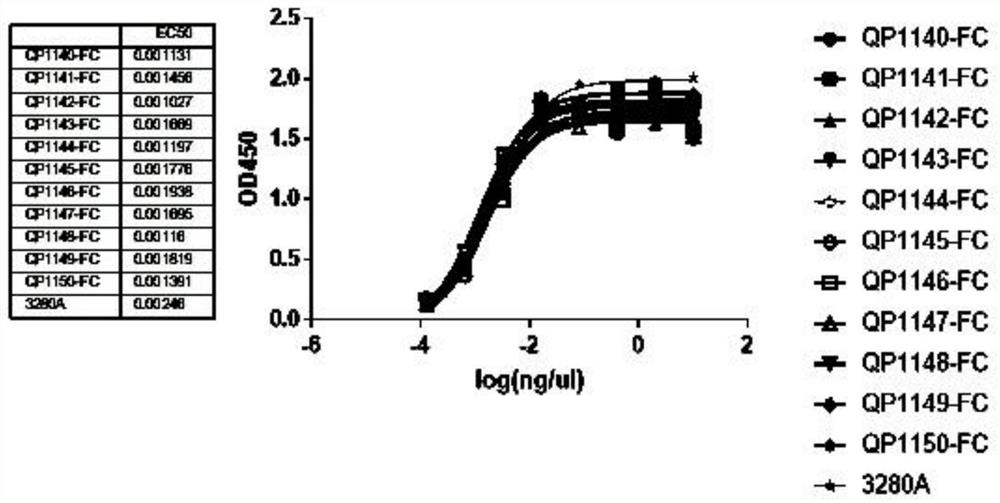
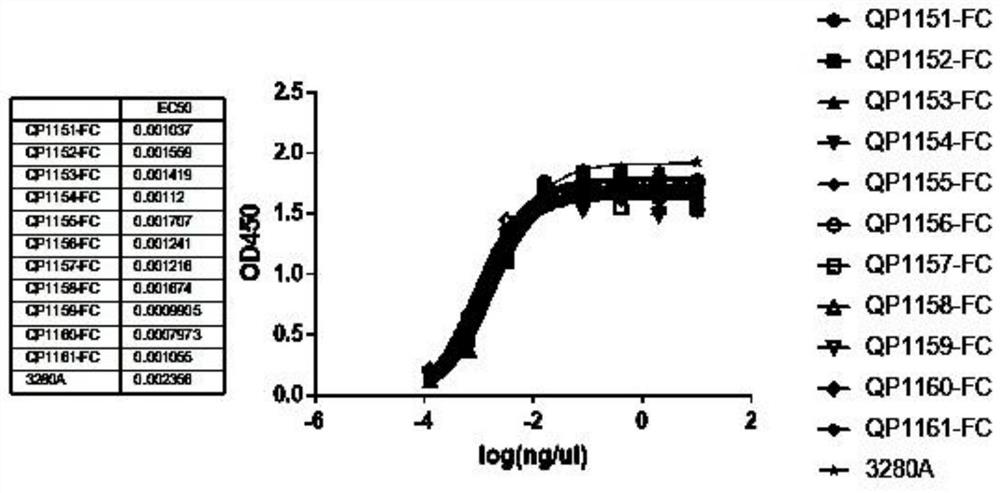
Anti-PD-L1 nano antibody as well as Fc fusion protein and application thereof
A PD-L1 and nanobody technology, applied in the field of biomedicine, can solve the problem of unsatisfactory affinity, and achieve the effect of high affinity, weak immunogenicity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation method of PD-L1 nanobody Fc fusion protein:
[0065] 1. Screening of nanobodies against PD-L1
[0066] 1.1 Library Construction
[0067] a) The PD-L1-his fusion protein of the immunized camel was obtained by purifying on a nickel column, the sequence of which is shown in SEQ ID NO.43. One Xinjiang Bactrian camel was selected for subcutaneous multi-point immunization, and 50 mL of peripheral blood was collected after four immunizations to separate PBMCs;
[0068] b) Total RNA was extracted with TRIzol reagent. After electrophoresis to identify sufficient RNA purity, use Super IIIFirst-Strand Synthesis System for RT-PCR transcribed 8ug RNA, and after 2 rounds of nested PCR, recovered and purified the target nucleic acid fragments with a DNA product purification kit.
[0069] c) The phage vector pComb3XSS and the target fragment were respectively digested with sfiI, digested overnight at 50°C, and then the target fragment was recovered by tapping t...
Embodiment 2
[0161] Example 2 Humanization of Anti-PD-L1 Nanobodies
[0162] By comparing the IMGT human antibody heavy and light chain variable region germline gene database and MOE software, the heavy and light chain variable regions with high homology to QP1162 (SEQ ID No.35) and QP1166 (SEQ ID No.39) were selected respectively. The region germline gene was used as a template, and the CDRs of the mouse antibody were transplanted into the corresponding human templates to form a variable region sequence in the order of FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4. Then select some important amino acid residues for back mutation combination. The amino acid residues are identified and annotated by the Kabat numbering system.
[0163] Primer PCR was designed to build the VH gene fragments of each humanized antibody, and then homologous recombination was performed with the expression vector pQD with signal peptide and constant region gene (FC) fragments to construct the full-length expression vector VH-FC...
Embodiment 3
[0192] Example 3 Activity identification of humanized anti-PD-L1 nanobody
[0193] Construct and design anti-CLDN18.2 / anti-PD-L1 bispecific antibody molecule QP3711461, in the form of Figure 8 shown.
[0194]
[0195] 1. In vitro activity identification of PD-L1 function (mixed lymphocyte reaction MLR)
[0196] Prepare DC (donor1) cells: recover PBMC, use EasySep TM Human Monocyte Isolation Kit (Stemcell 19359) was used to isolate mononuclear cell monocytes, add rhGM-CSF (1000U / ml) and rhIL4 (500U / ml), and culture the cells at 37°C for 6 days to induce iDCs; every 2-3 days half change medium, Supplement rhGM-CSF (1000U / ml) and rhIL4 (500U / ml) at the same time; collect the cells and centrifuge at 300xg for 5 minutes, resuspend with the medium added with rhGM-CSF (1000U / ml) and rhIL4 (500U / ml), and add LPS at the same time (1 μg / ml), continue to culture the cells at 37°C for 1 day to induce mature DCs; collect the cells and count them for later use.
[0197] Prepare T(do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com