Immunoassay kit for detecting M-type phospholipase A2 receptor-IgG as well as preparation method and detection method of immunoassay kit
An immunoassay and kit technology, applied in immunoassay assay technology and nano-biology field, can solve the problem of easy recurrence, achieve the effects of no radioactive contamination, shorten the reaction time, and improve the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] The present invention will be further illustrated below through specific implementation examples. However, these examples are only for illustrating the present invention and are not intended to limit the scope of the present invention.
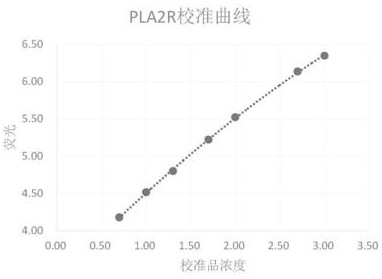
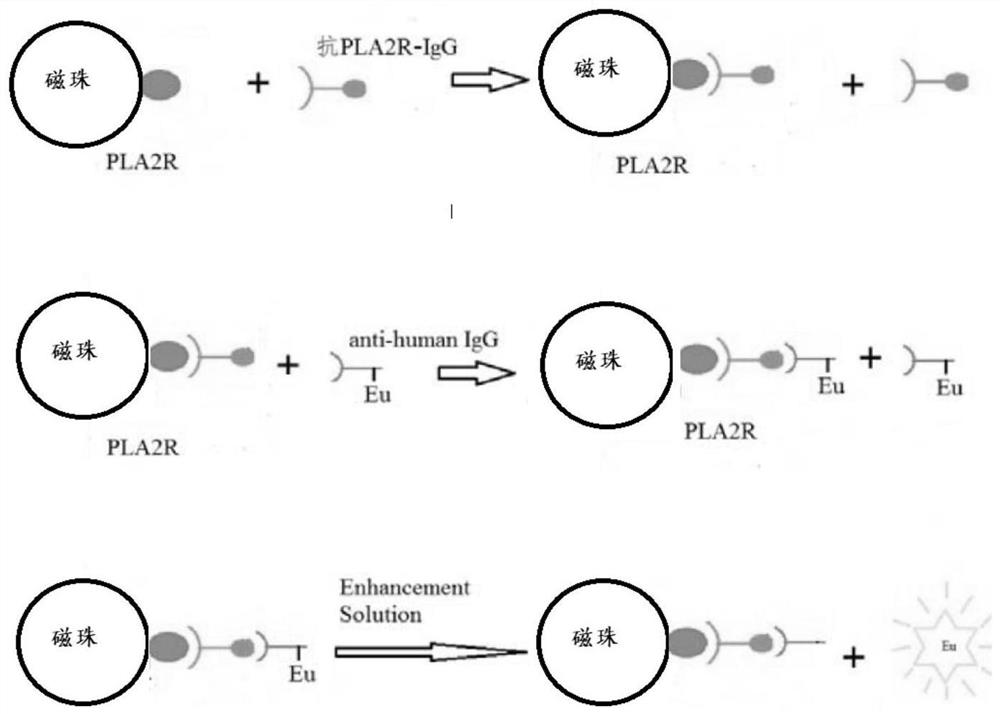
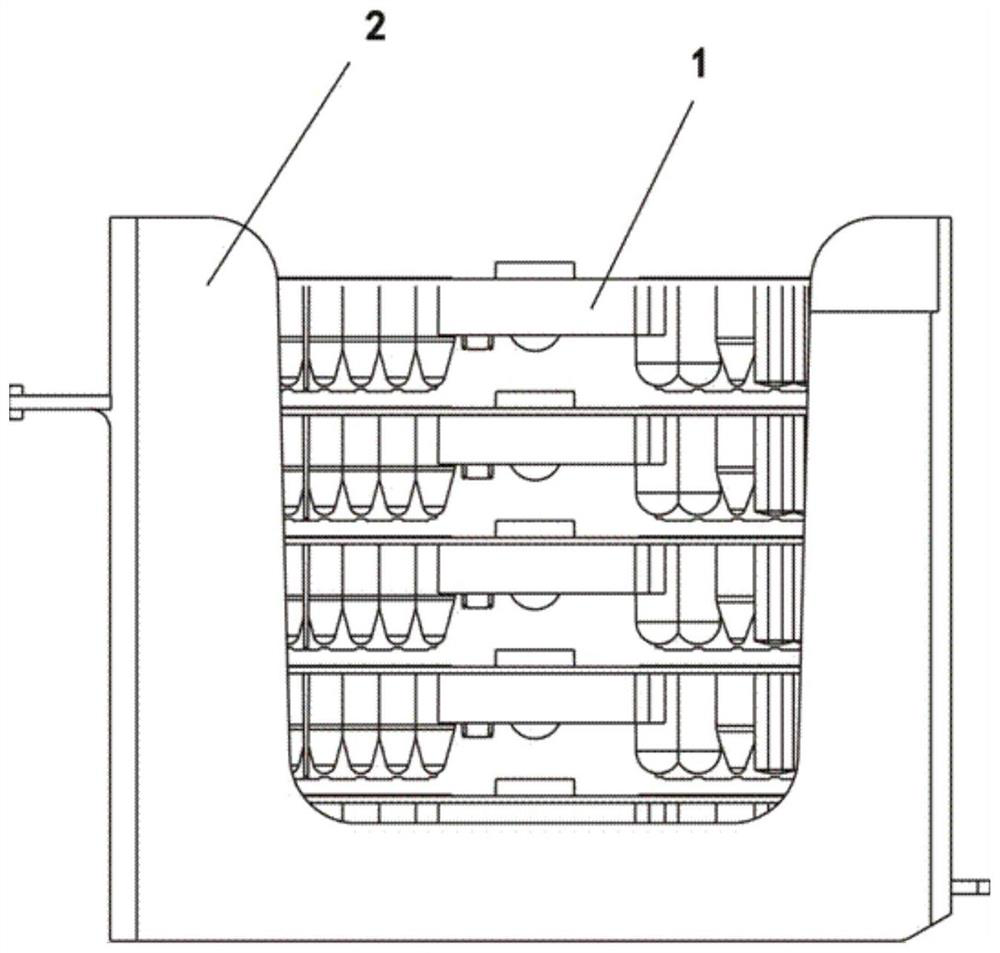
[0035] Such as image 3 with 4 As shown, an immunoassay kit for detecting M-type phospholipase A2 receptor-IgG, the kit includes 5 to 20 reagent racks, and each of the reagent racks is provided with 5 reagent strips; The kit also includes 2 vials of anti-PLA2R-IgG lyophilized calibrator. The reagent strip includes magnetic bead wells, reaction buffer wells, europium-labeled freeze-dried wells, detection wells, cleaning solution wells, enhancement solution wells and several reserved wells; the magnetic bead wells are filled with PLA2R antigen-coated Magnetic particle solution, the reaction buffer hole is filled with reaction buffer, the europium-labeled freeze-dried hole is filled with europium-labeled goat anti-human IgG antibody sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com