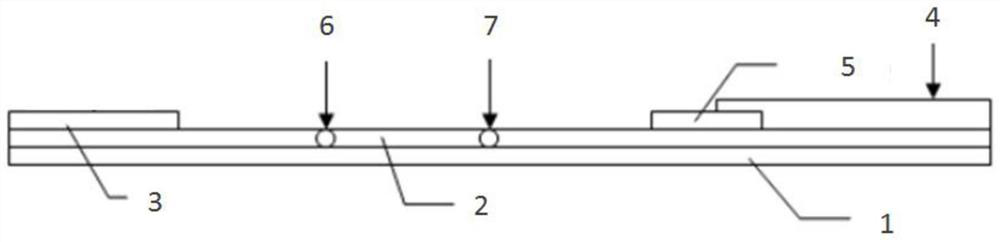
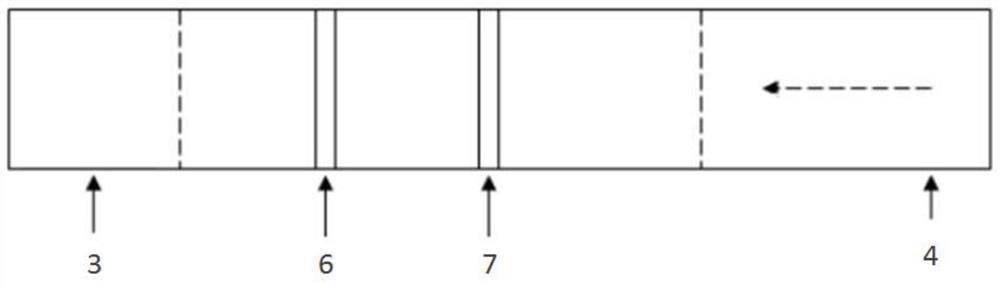
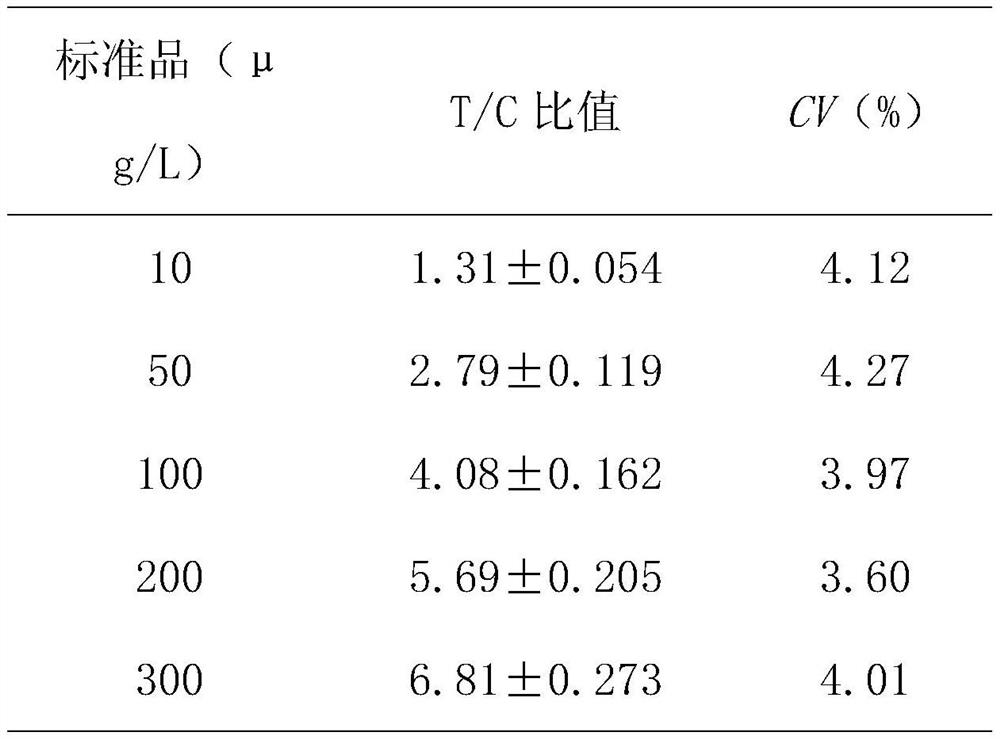
A method for rapid intraoperative identification of cervical lymph node metastasis in papillary thyroid carcinoma
A technology for lymph node metastasis and papillary carcinoma, applied in instruments, measuring devices, scientific instruments, etc., can solve the problems of low contrast between color strips and background, inability to realize quantitative detection of test objects, and difficulty in realizing multiple inspections and joint inspections, etc. To achieve the effect of reducing the incidence of hypoparathyroidism, reducing external conditions and background, and shortening the length of hospital stay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Screening of human Tg antigen peptides
[0046] The Tg described in this application is known in the art, and a complete Tg is composed of a single polypeptide chain containing 2768 amino acids, with a molecular weight of about 660,000 Daltons. Its amino acid sequence is known in the art and can be found in professional databases such as NCBI, and the specific sequence is as follows:
[0047] Human Tg(1-2768):
[0048] MALVLEIFTLLASICWVSANIFEYQVDAQPLRPCELQRETAFLKQADYVPQCAED GSFQTVQCQNDGRSCWCVGANGSEVLGSRQPGRPVACLSFCQLQKQQILLSGYINSTDT SYLPQCQDSGDYAPVQCDVQQVQCWCVDAEGMEVYGTRQLGRPKRCPRSCEIRNRRLLH GVGDKSPPQCSAEGEFMPVQCKFVNTTDMMIFDLVHSYNRFPDAFVTFSSFQRRFPEVS GYCHCADSQGRELAETGLELLLDEIYDTIFAGLDLPSTFTETTLYRILQRRFLAVQSVISGRFRCPTKCEVERFTATSFGHPYVPSCRRNGDYQAVQCQTEGPCWCVDAQGKEMHGTR QQGEPPSCAEGQSCASERQQALSRLYFGTSGYFSQHDLFSSPEKRWASPRVARFATSCP PTIKELFVDSGLLRPMVEGQSQQFSVSENLLKEAIRAIFPSRGLARLALQFTTNPKRLQ QNLFGGKFLVNVGQFNLSGALGTRGTFNFSQFFQQLGLASFLNGGRQEDLAKPLSVGLD SNSSTGTPEAAKKDG...
Embodiment 2
[0053] Example 2: Preparation of TG antibodies
[0054] The TG epitope peptides (1) and (2) obtained in Example 1 were respectively connected with a carrier protein to prepare antigens (1) and (2) for immunization, and the obtained antigens (1) and (2) were used to immunize animals respectively, Thereby, specific monoclonal and polyclonal antibodies are prepared using the antigen (1), and specific monoclonal and polyclonal antibodies are prepared using the antigen (2).
[0055] 1. Preparation of antigens: Tg peptides were respectively connected with carrier protein BSA to prepare TG antigens. Take 5.0 mg of each of the two antigenic peptides described in this application, dissolve them in 1 mL of DMF, and add 5 mg of EDC (dissolved in 50 μL of H) dropwise. 2 O), after stirring for 20 min, the reaction was added dropwise to 5 mg of BSA (dissolved in 1 mL of PBS buffer), 5 mg of NHS was added immediately, and the mixture was stirred at room temperature overnight. The reaction ...
Embodiment 3
[0067] Example 3: Specific identification of human TG antibodies (1) and (2)
[0068] Detected by ELISA. ELISA plates were coated with human Tg protein, GAPDH protein, and neuron-specific enolase NSE as detection antigens, respectively, and the specific reactions of the prepared TG monoclonal antibodies (1) and (2) with different proteins were detected by ELISA. , normal BALB / c mouse serum was used as negative control, and PBS solution was used as blank control.
[0069] Results: Tg monoclonal antibodies (1) and (2) only reacted positively with Tg (P / N > 2.1), respectively, but were negative with GAPDH protein and neuron-specific enolase NSE, indicating that the antibody of the present invention was used. The monoclonal antibodies (1) and (2) prepared from the Tg epitope peptides (1) and (2) have specificity.
[0070] Polyclonal antibodies were identified using the same methods described above for identifying the specificity of monoclonal antibodies.
[0071] The results sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com