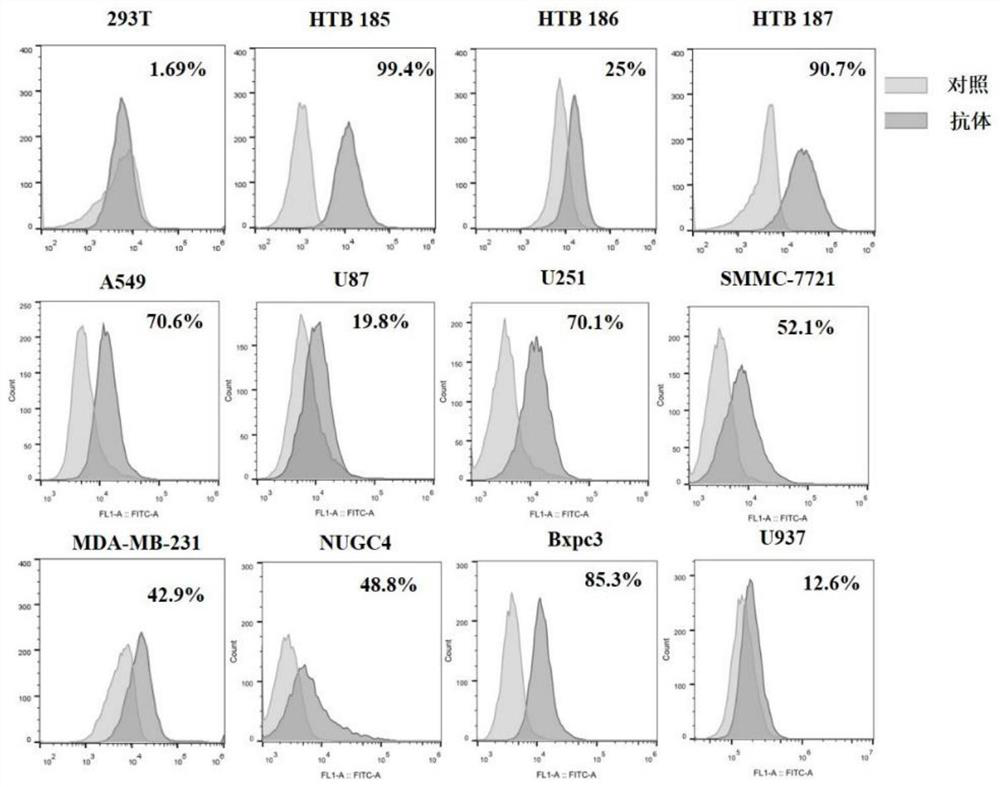
Siglec-15 monoclonal antibody and applications thereof
A monoclonal antibody, recombinant vector technology, applied in the application, antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin and other directions, can solve the problem of immune system detection difficulties and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Obtaining of human Siglec-15 hybridoma cell line and preparation of monoclonal antibody
[0045] 1) Animal immunity
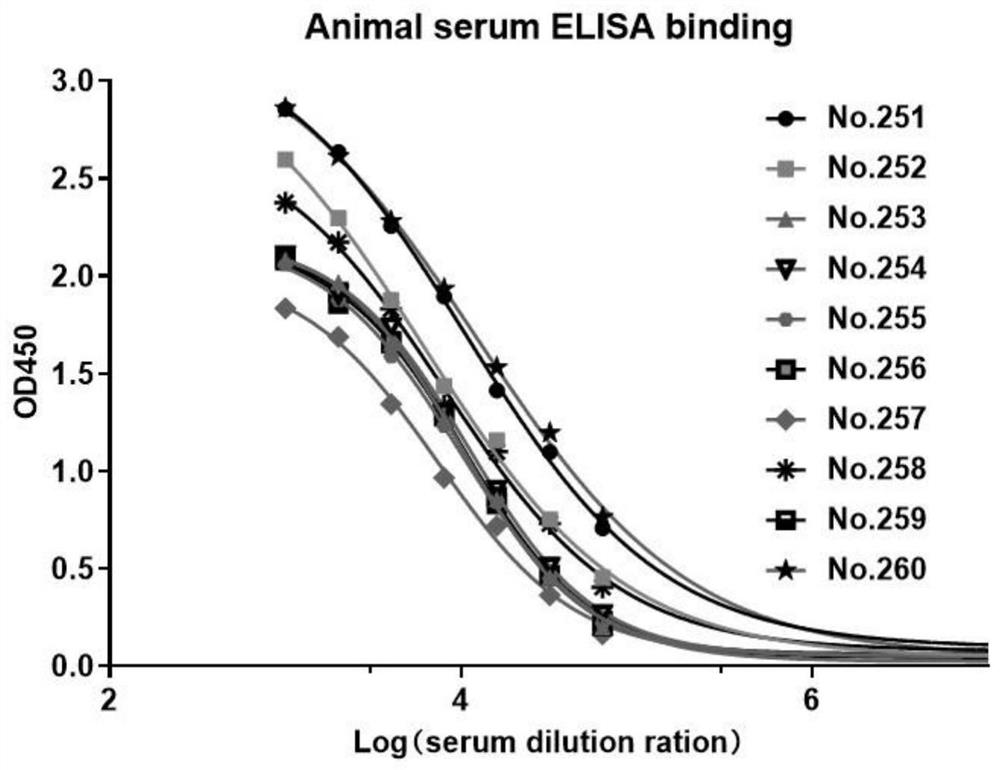
[0046] The antigen uses the recombinant protein Siglec-15-Fc fused to the human Siglec-15 extracellular domain of the human IgG1 Fc segment. Female Balb / c and C57bl / 6 mice were immunized subcutaneously with 50 μg Siglec-15-Fc fusion protein in 200 μl Freund's complete adjuvant (Sigma-Aldrich) in a 1:1 emulsion. Mice were subsequently boosted with alternating ip / sc injections of 25 μg Siglec-15-Fc in a 1:1 emulsion in Freund's incomplete adjuvant (Sigma-Aldrich) up to 3 times every two weeks. Serum titers of 10 mice all reached 10 after three immunizations 5 above. 4 days before myeloma fusion, showing the highest antibody titer ( figure 1 ) of two mice (No. 251 and No. 260) received an intraperitoneal booster immunization with 25 μg Siglec-15-Fc (without adjuvant).
[0047] 2) Hybridoma fusion and screening
[0048] Spleens were extracte...
Embodiment 2
[0052] Example 2: Production and purification of hybridoma sequencing and antibody
[0053] After using the fast ELISA mouse antibody subtype identification kit (Clono typing System-HRP, SouthernBiotech) to identify the subtype of the monoclonal antibody, use TRIzol (Ambion) from 3 × 10 6 ~5×10 6 Total RNA was extracted from hybridoma cells, and antibody subtype-specific primers and universal primers (PrimeScript TM 1stStrand cDNASynthesis Kit, Takara) to reverse transcribe it into cDNA. Murine immunoglobulin heavy and light chain V-region fragments were subsequently amplified by RACEPCR and the resulting PCR fragments were subcloned into the pMD18-T vector system (Takara) and the inserts were sequenced using vector-specific primers. Finally, the heavy chain and light chain protein sequences of clones C9, C18, C30, C31, C40, C46, and C52 were obtained.
[0054] Table 1 is the sequence information of the heavy chain complementary region:
[0055]
[0056] Table 2 is t...
Embodiment 3
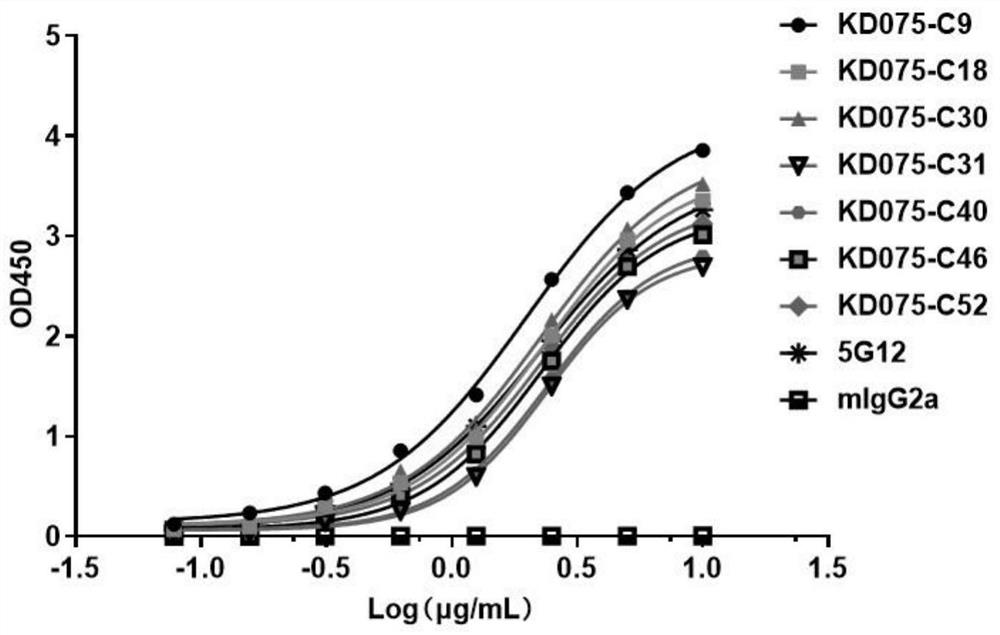
[0061] Example 3: Binding of monoclonal antibody to human Siglec-15 recombinant protein
[0062] Indirect ELISA was used to assess the binding ability of purified antibodies to Siglec-15-Fc. ELISA plates (Nunc) were coated with 100 μl / well of 0.5 μg / ml recombinant Siglec-15-Fc or human IgG1 in PBS overnight at 4°C. Plates were washed with PBS-T (0.05% Tween), and blocked with 200 μl / well of 1% BSA in PBST at 37° C. for 0.5 hours. Then discard the blocking solution, add 100 μl of 10 μg / ml purified antibody to the first well, and dilute according to 2-fold gradient, a total of 8 test concentration gradients. Then incubate for 1 hour at room temperature. Plates were washed three times with PBST and incubated with 100 μl / well horseradish peroxidase-conjugated goat anti-mouse IgG (Fab-specific) for 0.5 hours at 37°C. Plates were washed five times with PBST, then TMB chromogenic solution was added and incubated for 15 minutes at room temperature in the dark. The reaction was sto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com