Higher-functionality antihuman CTLA4 antibody with high affinity and high specificity as well as multi-antigen recognition epitopes
A technology of expressing vectors and monoclonal antibodies, which is applied in the fields of tumor immunotherapy and molecular immunology, can solve problems such as side effects and achieve a large variety of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Obtaining of human CTLA4 hybridoma cell line
[0046] 1) Animal immunity
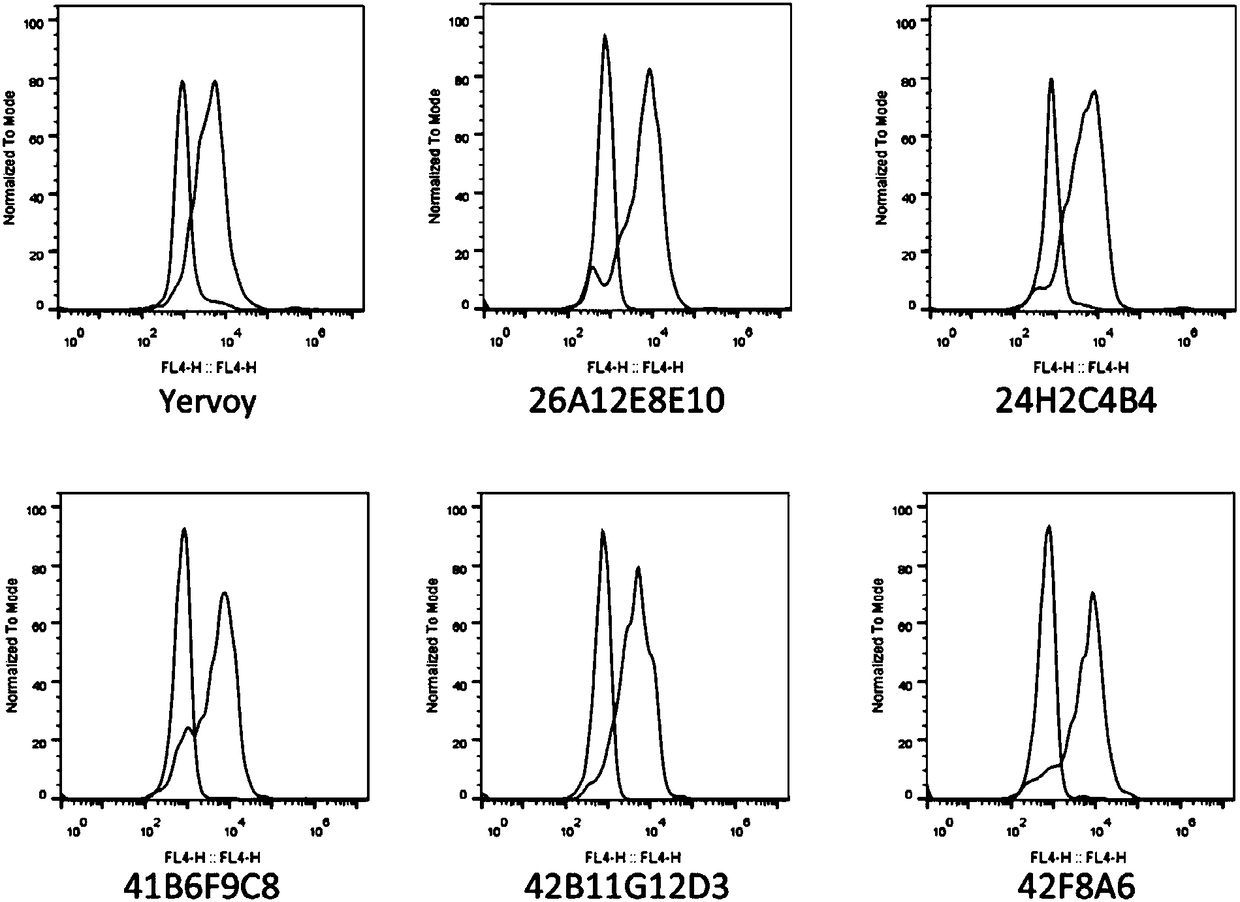
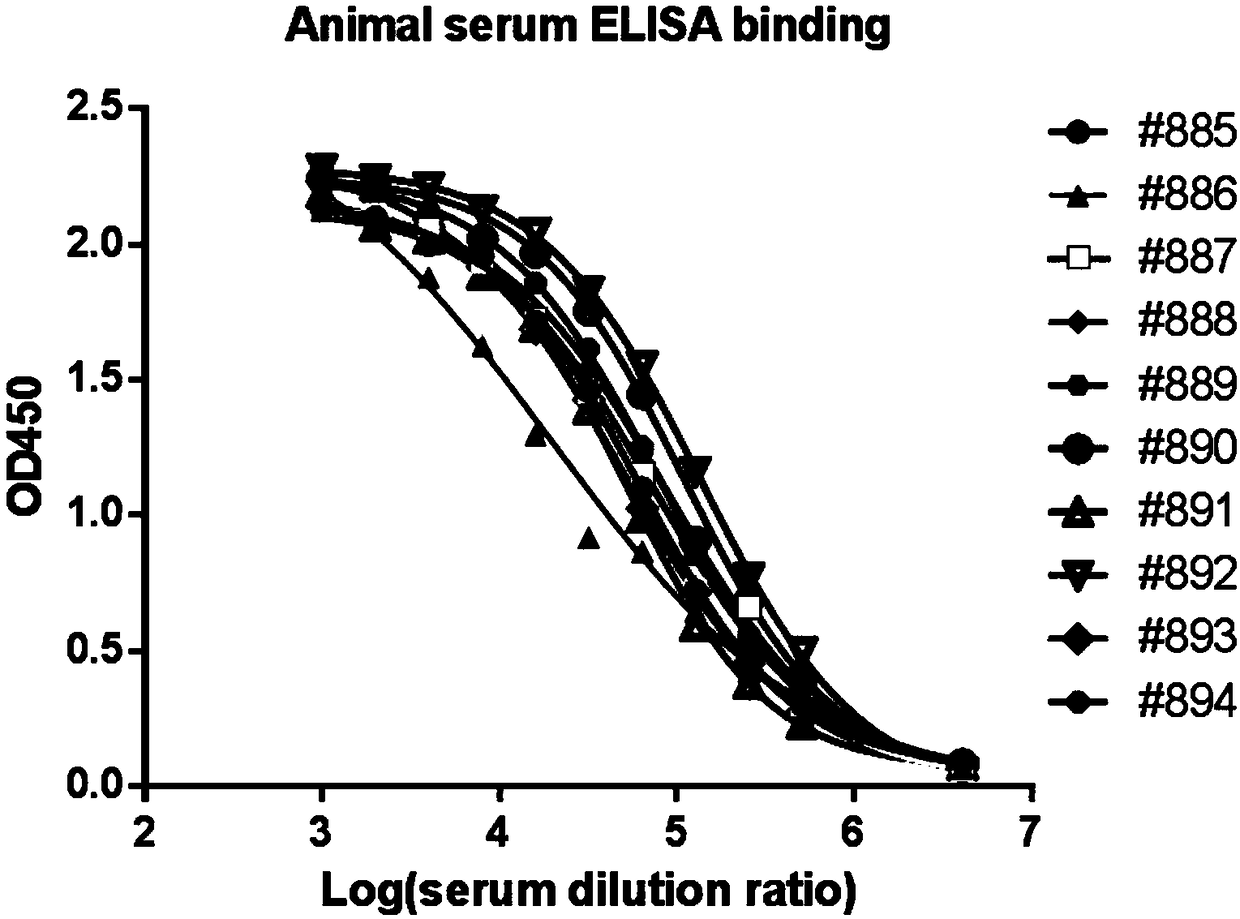
[0047] As the antigen, a recombinant protein CTLA4-Fc (GenScript, Z03373) fused to the human CTLA4 extracellular domain of the human IgG1 Fc fragment was used. Female Balb / c and C57bl / 6 mice were immunized subcutaneously with 50 μg CTLA4-Fc fusion protein in 200 μl Freund's complete adjuvant (Sigma-Aldrich) in a 1:1 emulsion. Mice were then boosted with alternating ip / sc injections of 25 μg CTLA4-Fc in a 1:1 emulsion in Freund's incomplete adjuvant (Sigma-Aldrich) every two weeks up to 3 times. 4 days before myeloma fusion, showing the highest antibody titer ( figure 1 ) received an intraperitoneal boost of 25ug CTLA4-Fc (without adjuvant).
[0048] 2) Hybridoma fusion and screening
[0049] Spleens were extracted and homogenized to generate single cell suspensions, while myeloma cell (SP2 / 0) single cell suspensions were prepared. The 8.9×10 7 splenocytes with 4.1×10 7 SP2 / 0 ...
Embodiment 2
[0055] Example 2: Variable region sequencing of monoclonal antibodies and antibody recombinant production
[0056] After using the rapid ELISA mouse antibody subtype identification kit (Clonotyping System-HRP, SouthernBiotech) to identify the subtype of the antibody in the hybridoma cell culture supernatant, use TRIzol (Ambion) from 3 × 10 6 -5×10 6 Total RNA was extracted from hybridoma cells, and antibody subtype-specific primers and universal primers (PrimeScript TM 1stStrand cDNA Synthesis Kit, Takara) to reverse transcribe it into cDNA. Murine immunoglobulin heavy and light chain V-region fragments were subsequently amplified by RACE PCR (GenScript), and the resulting PCR fragments were subcloned into the pMD18-T vector system (Takara) and inserted using vector-specific primer pairs Fragments are sequenced. Finally, unique V-region nucleotide / protein sequences of clones 26A12E8, 24H2C4B4, 42B11G12D3, 41B6F9C8, 42F8A6 were obtained.
[0057] Amino acid sequence of 26A...
Embodiment 3
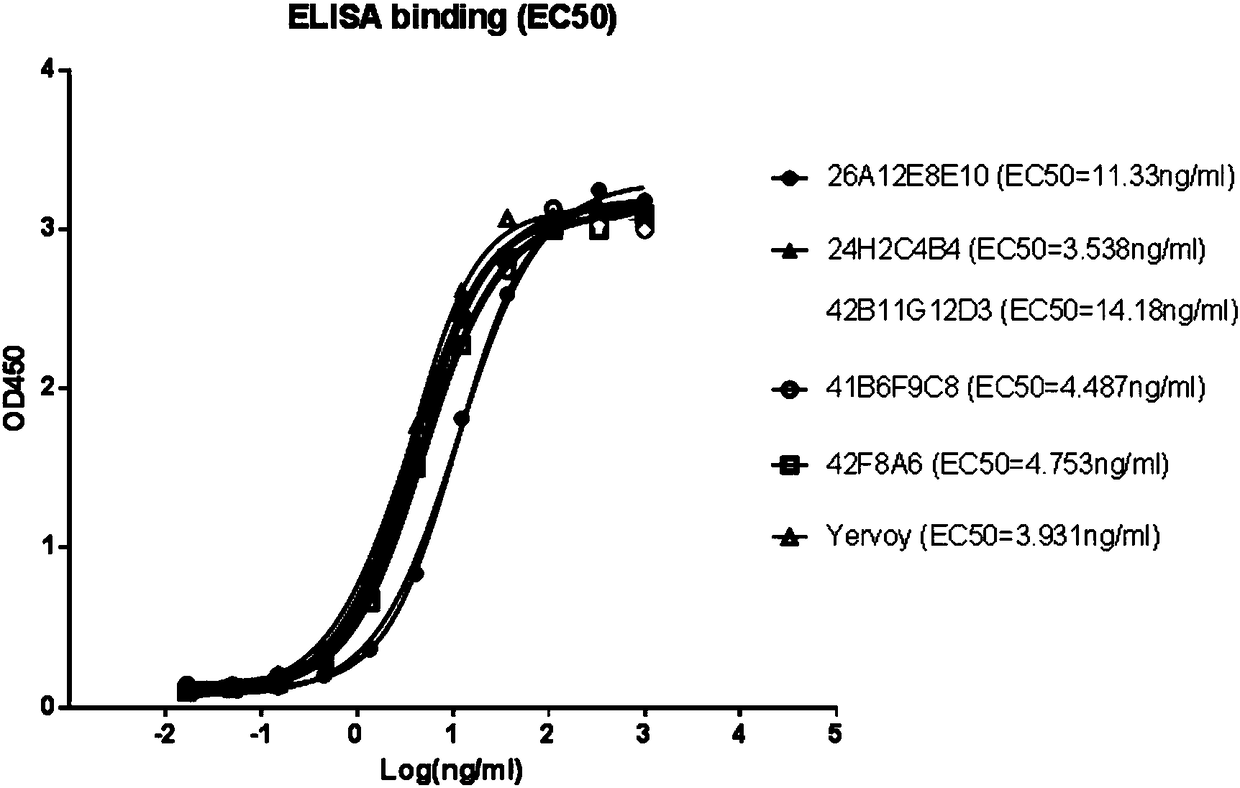
[0080] Example 3: Binding of monoclonal antibodies to human CTLA4 recombinant protein
[0081] Indirect ELISA was used to assess the binding ability of purified antibodies to CTLA4-Fc. ELISA plates (Nunc) were coated with 100 μl / well of 0.5 μg / ml recombinant CTLA4-Fc or human IgG1 in PBS overnight at 4°C. Plates were washed with PBS-T (0.05% Tween), and blocked with 200 μl / well of 1% BSA in PBST at 37° C. for 0.5 hours. Then discard the blocking solution, add 100 μl of 10 μg / ml purified antibody to the first well, and dilute according to 3-fold gradient, a total of 11 test concentration gradients. Then incubate for 1 hour at room temperature. Plates were washed three times with PBST and incubated with 100 μl / well horseradish peroxidase-conjugated goat anti-mouse IgG (Fab-specific) (GenScript) for 0.5 hours at 37°C. Plates were washed five times with PBST, then TMB Chromogenic Solution (GenScript) was added and incubated for 15 minutes at room temperature in the dark. The r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com