Method for removing alpha '-crystal form aluminum hydride
A technology of aluminum hydride and crystal form, which is applied in the field of aluminum hydride and α′ crystal form aluminum hydride removal, can solve the problem of incomplete removal of α’ crystal form aluminum hydride, and achieve simple and easy operation of the reaction device, solvent The effect of small amount and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment provides a method for removing α' crystal form aluminum trihydride, which is carried out according to the following steps:
[0038] Step 1, under an inert dry atmosphere, take aluminum trihydride to be purified, add solvent toluene, and mix evenly by ultrasonic waves;
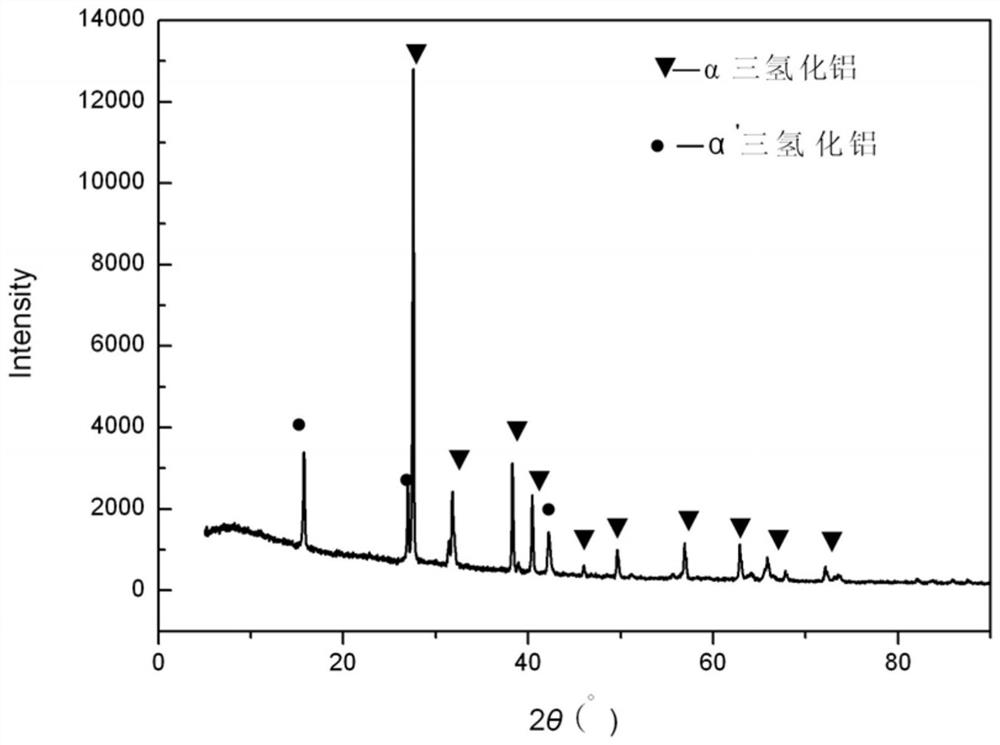
[0039] The purity of the aluminum trihydride to be purified is 99.0wt.%, that is, it contains 1.0wt.% of α' crystal form aluminum trihydride.
[0040] The mass volume ratio of the aluminum trihydride to be purified and the solvent is: 0.8g: 16ml;
[0041] Step 2, transfer the uniformly mixed solution in step 1 to the reactor, seal the reactor, place the reactor in an oven and raise the temperature to 140°C, and react at a constant temperature for 8 hours;
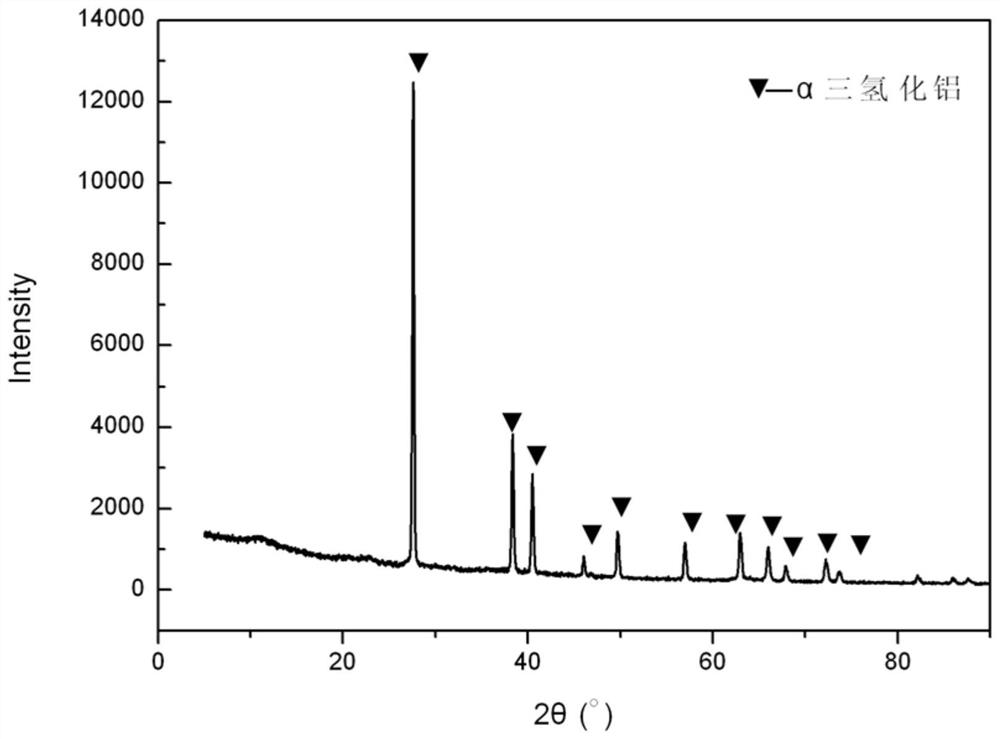
[0042] In step 3, after the reaction is finished, cool naturally and filter, and the product obtained is pure-phase α-crystal form aluminum trihydride.
[0043] Product Purity Analysis:
[0044] figure 1 It is the XRD pattern of al...
Embodiment 2
[0048] This embodiment provides a method for removing α' crystal form aluminum trihydride, which is carried out according to the following steps:
[0049] Step 1, under an inert dry atmosphere, take aluminum trihydride to be purified, add solvent toluene, and mix evenly by ultrasonic waves;
[0050] The purity of the aluminum trihydride to be purified is 99.0wt.%, that is, it contains 1.0wt.% of α' crystal form aluminum trihydride.
[0051] The mass volume ratio of the aluminum trihydride to be purified and the solvent is: 0.5g: 15ml;
[0052] Step 2, transfer the uniformly mixed solution in step 1 to the reactor, seal the reactor, place the reactor in an oven and raise the temperature to 100°C, and react at a constant temperature for 10 hours;
[0053] In step 3, after the reaction is finished, cool naturally and filter, and the product obtained is pure-phase α-crystal form aluminum trihydride.
[0054] The product purity analysis of this embodiment is basically the same as...
Embodiment 3
[0056] This embodiment provides a method for removing α' crystal form aluminum trihydride, which is carried out according to the following steps:
[0057] Step 1, under an inert dry atmosphere, take aluminum trihydride to be purified, add solvent toluene, and mix evenly by ultrasonic waves;
[0058] The purity of the aluminum trihydride to be purified is 99.0wt.%, that is, it contains 1.0wt.% of α' crystal form aluminum trihydride.
[0059] The mass volume ratio of the aluminum trihydride to be purified and the solvent is: 1.0g: 18ml;
[0060] Step 2, transfer the uniformly mixed liquid in step 1 to the reactor, seal the reactor, place the reactor in an oven and raise the temperature to 160°C, and react at a constant temperature for 6 hours;
[0061] In step 3, after the reaction is finished, cool naturally and filter, and the product obtained is pure-phase α-crystal form aluminum trihydride.
[0062] The product purity analysis of this embodiment is basically the same as th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com