Composite preparation capable of effectively inhibiting HIV, HBV, H1N1, H3N2 and nCoV
A compound preparation, H3N2 technology, applied in the direction of medical preparations containing active ingredients, antiviral agents, organic active ingredients, etc., can solve the problems of side effects, inability to stop drug at will, reduce damage, improve autoimmune system, slow down speed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Experimental purpose: to determine the inhibition of the compound preparation on HBV.
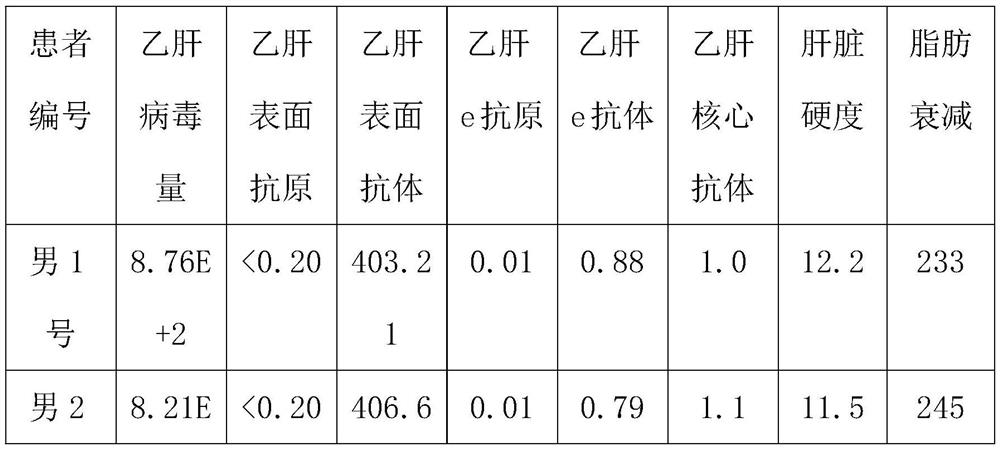
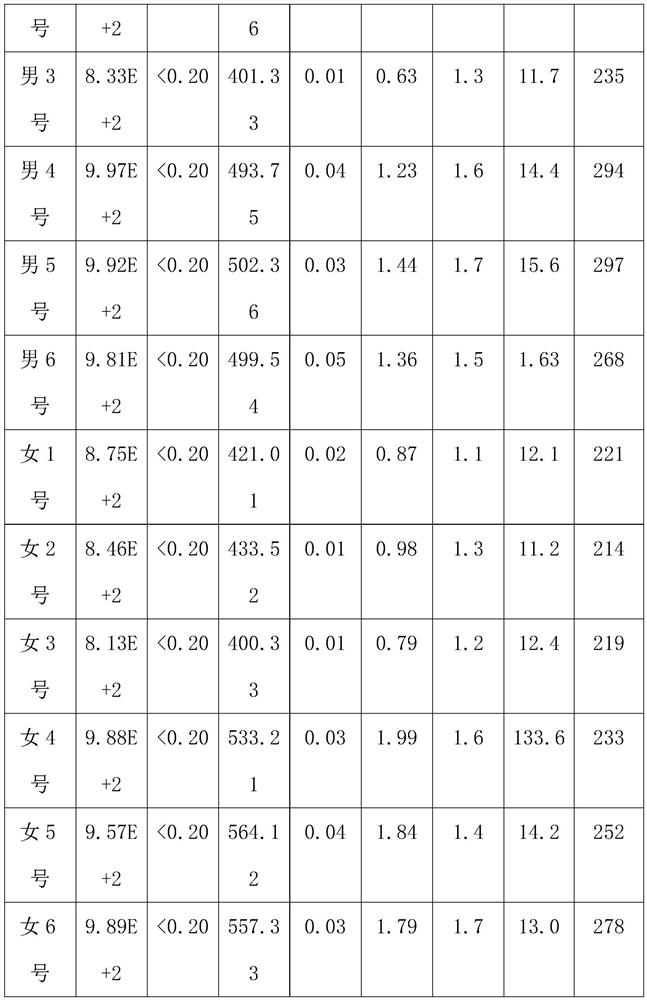
[0032] Subjects: 12 patients, including 6 male patients and 6 female patients, aged between 30 and 35, all of them are hepatitis B virus carriers.
[0033] The patient's condition: the value of hepatitis B virus DNA is 9.09E+2, the hepatitis B surface antigen is greater than 0.2ng / ml, the hepatitis B surface antibody is 460-480IU / L, the hepatitis B e antigen is 0.6-0.7PEIU / mL, and the hepatitis B e antibody is 1.02 -1.1PEIU / mL, hepatitis B core antibody is 1.3-1.5PEIU / mL, median of liver stiffness (KPA) is 13.3-13.4, median of fat decay (db / m) is 265-295, color Doppler ultrasound result is normal .
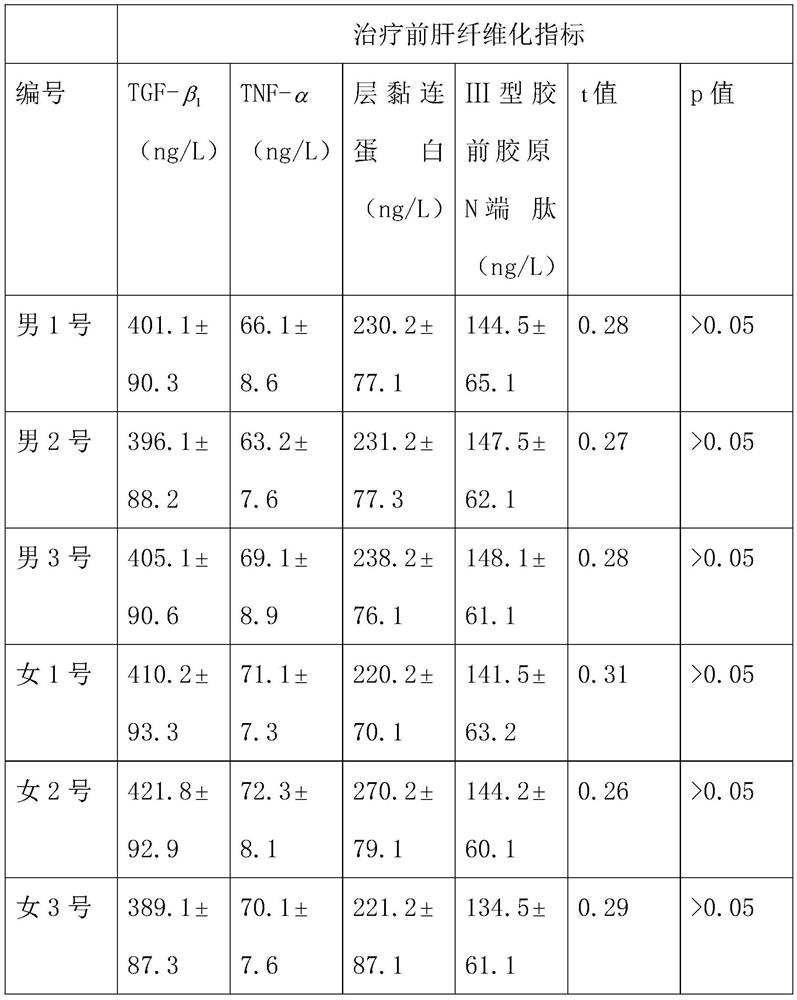
[0034] Experimental method: 3 male patients, male No. 1, male No. 2 and male No. 3, and 3 female patients, female No. 1, female No. 2 and female No. 3, were selected to take the compound preparation respectively for a period of 8 months. The unselected 3 male patients, male No. 4, male...
Embodiment 2
[0047] Experimental purpose: To determine the inhibition of the compound preparation on nCoV.
[0048] Experimental objects: 12 pigs were selected, of which 6 were adult boars and 6 were juvenile boars, all infected with the novel coronavirus (nCoV).
[0049] Experimental method: Select adult boar No. 1, adult boar No. 2, adult boar No. 3, juvenile boar No. 1, juvenile boar No. 2 and juvenile boar No. 3 to take the compound preparation, adult boar No. 4 , No. 5 adult boar, No. 6 adult boar, No. 4 juvenile boar, No. 5 juvenile boar and No. 6 juvenile boar did not take the compound preparation, but carried out the same feeding, and the survival of 12 pigs To record, the recording period is 10 days, and the records are shown in the following table:
[0050]
[0051]
[0052]As can be seen from the above table, the compound preparation can inhibit nCoV.
[0053] Principle of work of the present invention and use process:
[0054] In the present invention, the compound pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com