Solid preparation composition for oral administration of colonic purgative containing anhydrous sodium sulfate, potassium sulfate, anhydrous magnesium sulfate and simethicone
A technology of anhydrous magnesium sulfate and anhydrous sodium sulfate, which can be used in pharmaceutical combinations, active ingredients of silicon compounds, and medical preparations containing active ingredients, etc. Unpleasant odor, efficacy in improving medication adherence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
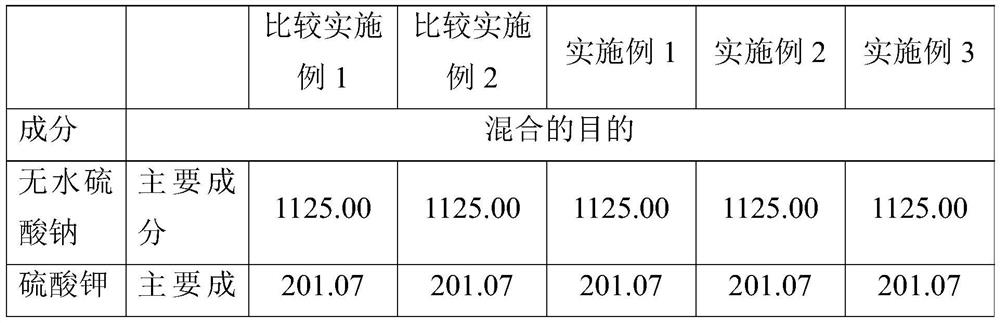
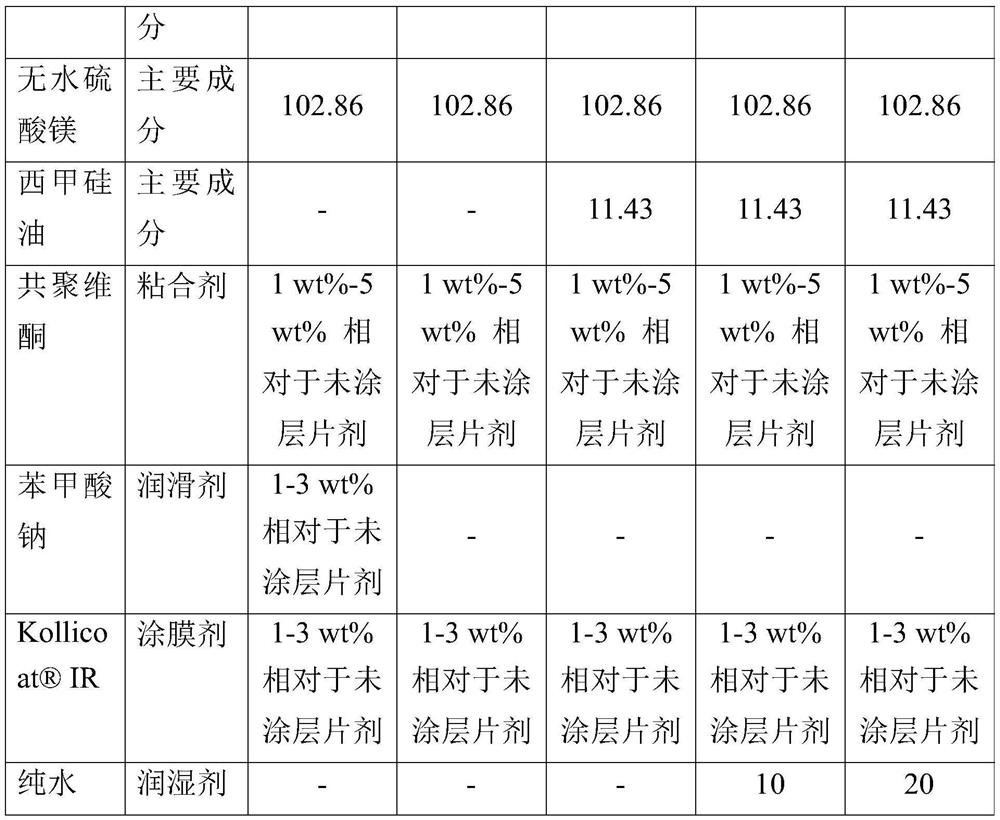
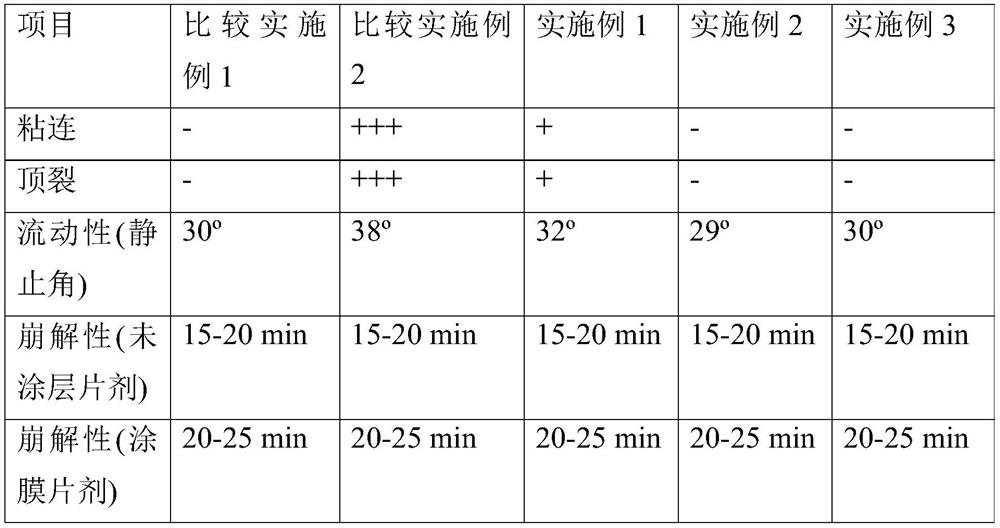
[0087] A comparison between Examples 1 and 2 reveals the effect of water. Example 1 of the present invention, even without water, is superior to Comparative Example 2 in properties related to blocking, capping, and fluidity, and thus is considered to be industrially feasible as required. However, adding only a small amount of water as in Example 2 increased flowability and reduced capping defects.
[0088] According to the addition amount of water, embodiment 2 and 3 have carried out process method comparison. Excess water caused no capping defects, but slightly more blocking defects (however, less than Comparative Example 2). For better productivity, the appropriate water content is 0.1wt%-2.0wt% per piece, preferably 0.5wt%-1.0wt% per piece.
[0089] In addition, there was no difference in disintegration time between Comparative Examples and Examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com