Preparation method of edoxaban tosylate and isomers thereof
A technology for toluenesulfonic acid and isomers, which is applied in the field of preparation of edoxaban tosylate and its isomers, and can solve problems such as the influence of drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
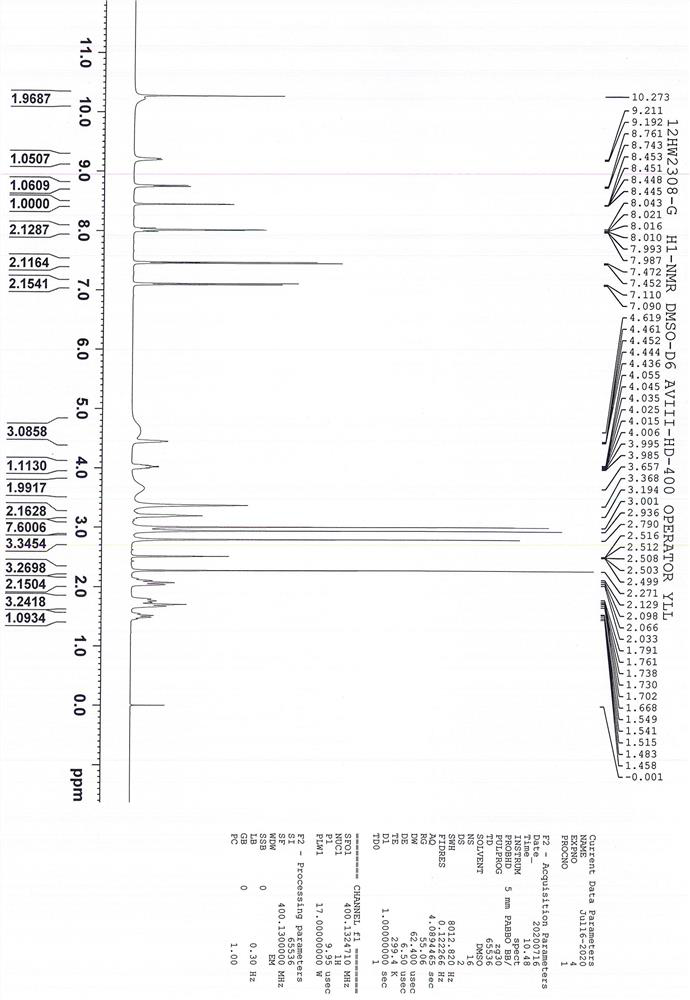
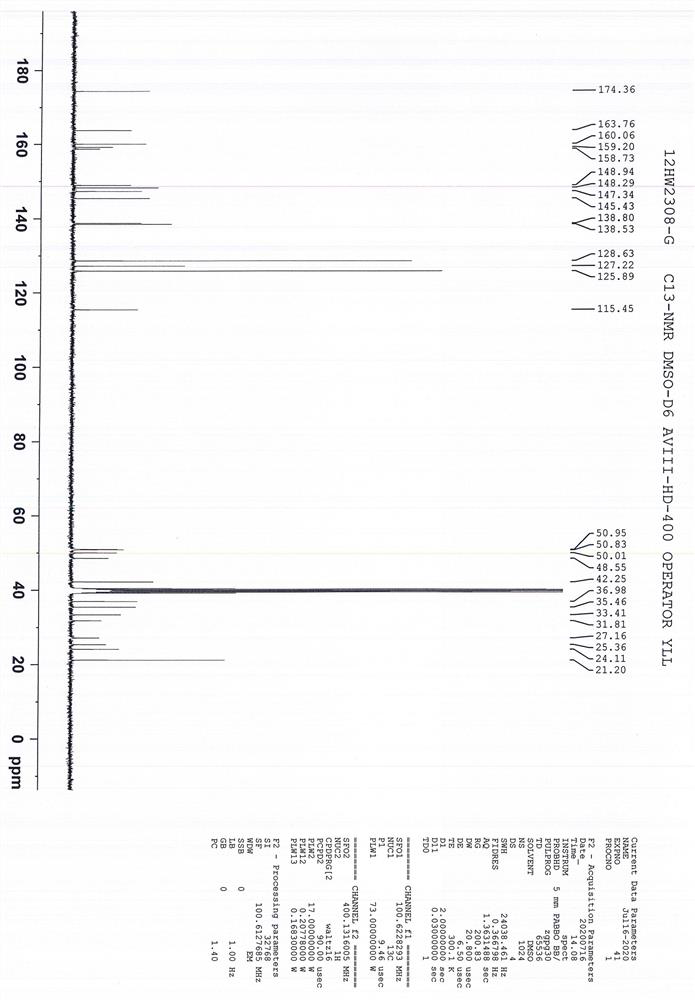
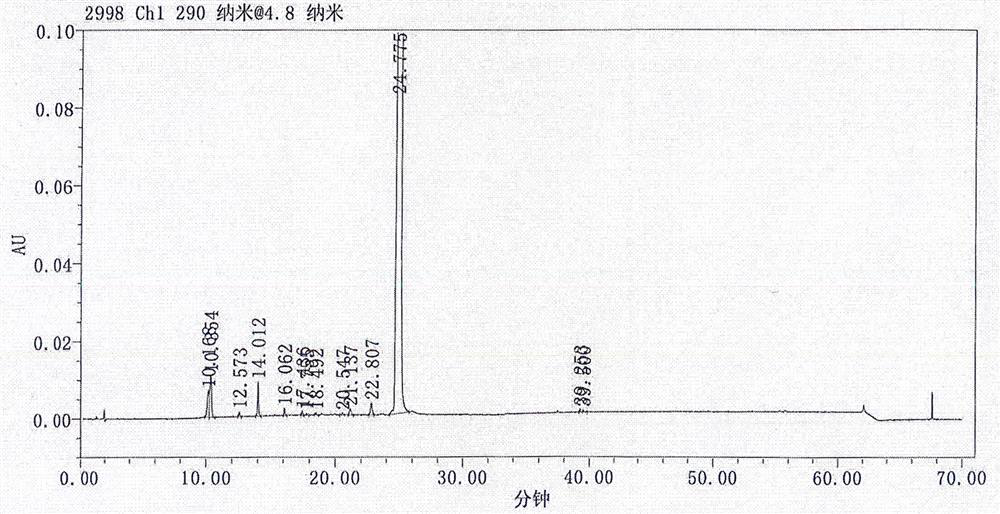
[0072] N 1 -(5-chloropyridin-2-yl)-N 2 -((1S,2R,4S)-4-((dimethylamino)carbonyl)-2-(((5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c] Pyridin-2-yl) formyl) amino) cyclohexyl) oxalamide p-toluenesulfonic acid monohydrate, i.e. the preparation method of edoxaban tosylate (1S, 2R, 4S), comprising the following steps:
[0073] S1. Add 200g of compound (I) to 1L of acetone, reflux and dissolve for 1h, dissolve 192.1g of compound (II) in 0.5L of acetone and add dropwise to the reaction solution, slowly cool down to 40°C for 2h, then cool down to Suction filtration at 20-25°C, blow-dry the filter cake at 40-45°C to obtain 270g of solid, add 8 times acetone for recrystallization, then cool down to 20-25°C for crystallization, suction filtration, blow-dry the filter cake at 40-45°C 170 g of compound (III) were obtained,
[0074]
[0075] S2. Add 170 g of compound (III) to 3 L of dichloromethane, add 3 L of 2N HCl solution and stir for 2 h, separate the layers, back-extract the aqueous ...
Embodiment 2
[0102] N1-(5-chloropyridin-2-yl)-N2-{(1R,2R,4S)-4-(dimethylaminocarbonyl)-2-[(5-methyl-4,5,6,7- Tetrahydrothiazolo[5,4-c]pyridin-2-yl)carboxamido]cyclohexyl}oxalamide p-toluenesulfonic acid monohydrate, i.e. (1R,2R,4S of edoxaban tosylate ) configuration of the diastereomer (epimer) preparation method, comprising the following steps:
[0103] Steps S1-S5 are the same as steps S1-S5 in the preparation method of edoxaban tosylate (1S, 2R, 4S);
[0104] S6, add 15g Add to 500ml of dichloromethane, add triethylamine, cool down and stir, drop the temperature down to 0°C, add 18g MSCl dropwise, stir at 20-25°C for 2 hours after the dropwise addition, drop down to 0°C, add 250ml 0.5N hydrochloric acid dropwise, dropwise add Add 500ml of dichloromethane for extraction and separation, wash the organic layer with 1L of purified water and 1L of saturated sodium chloride, dry with 50g of anhydrous magnesium sulfate, and concentrate the filtrate to dryness by suction filtration to obtai...
Embodiment 3
[0123] N1-(5-chloropyridin-2-yl)-N2-{(1R,2S,4R)-4-(dimethylaminocarbonyl)-2-[(5-methyl-4,5,6,7- Synthesis of tetrahydrothiazolo[5,4-c]pyridin-2-yl)carboxamido]cyclohexyl}oxalamide p-toluenesulfonic acid monohydrate, i.e. (1R,2S of edoxaban tosylate ,4R) The preparation method of the enantiomer of configuration comprises the following steps:
[0124] S1, with compound (I) and compound (II) as starting raw material, the chemical name of compound (I) is 3-cyclohexene-1-carboxylic acid, structural formula is Compound (II) is
[0125] Add 200g of compound (I) to 1L of acetone, reflux and dissolve for 1h, dissolve 192.1g of compound (II) in 0.5L of acetone and add dropwise to the reaction solution, slowly lower the temperature to 40°C for 2h, then cool down to 20~ Suction filtration at 25°C, blow-dry the filter cake at 40-45°C to obtain 270g solid, add 8 times acetone for recrystallization, then cool down to 20-25°C for crystallization, suction filtration, blow-dry the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com