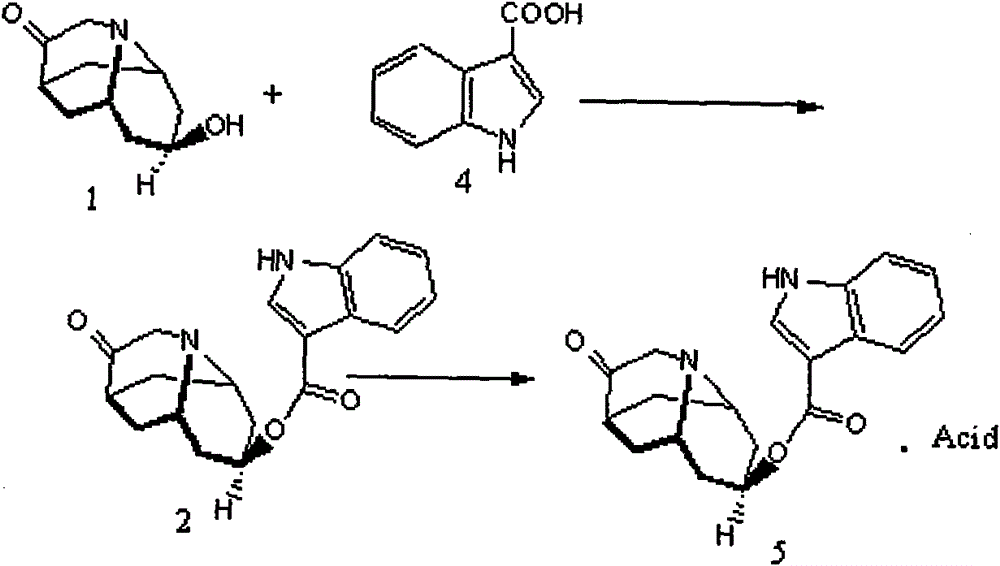
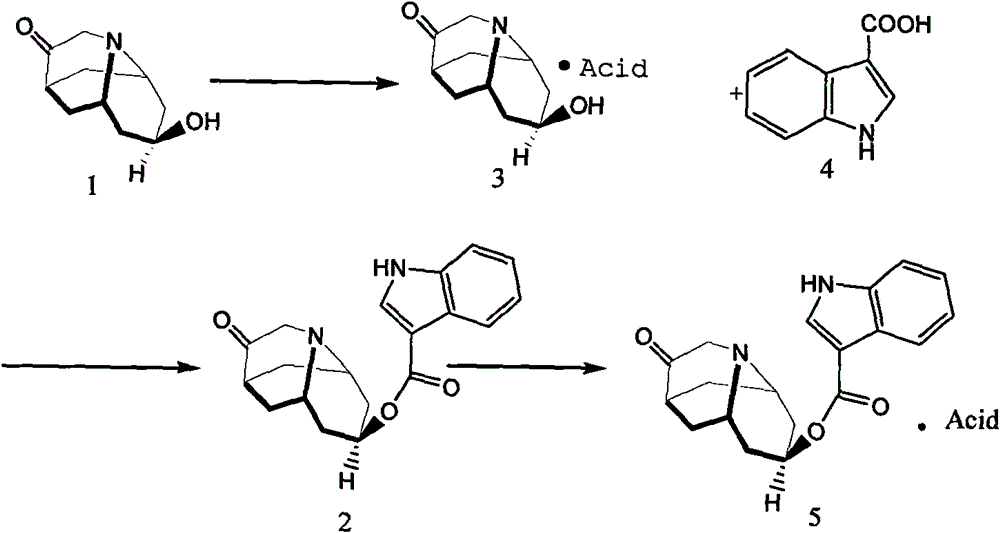
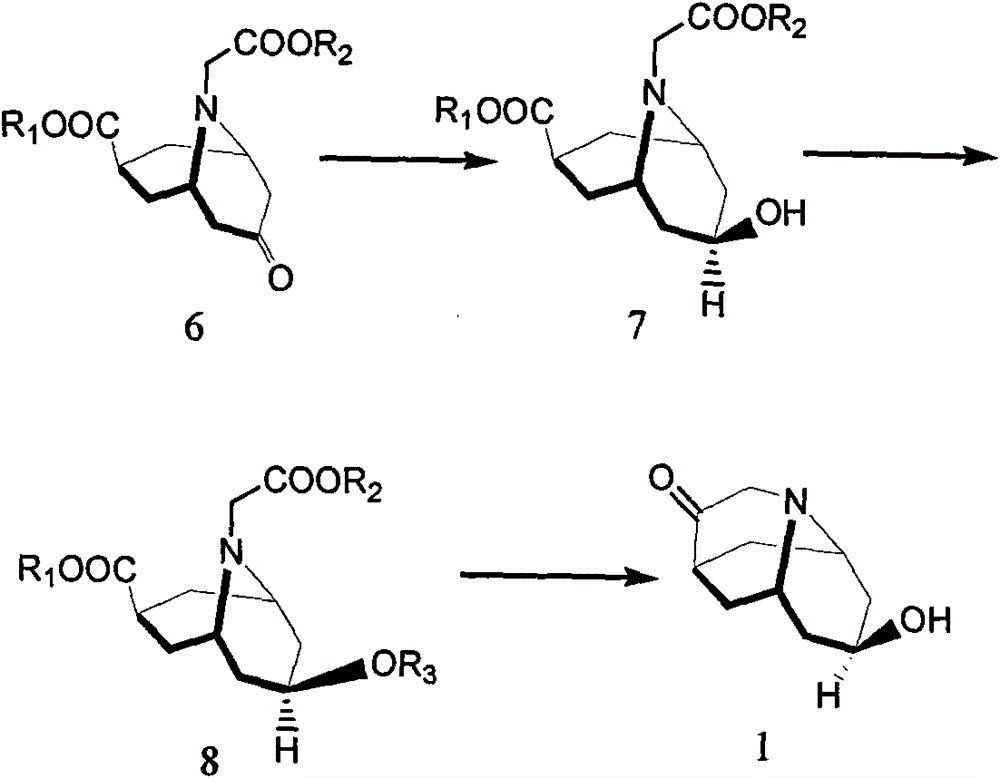
Method for detecting contents of dolasetron isomer and salt thereof
A technology of dolasetron and detection method is applied in the field of determination of drug content, and can solve the problems of preparation, confirmation, biological activity and detection method of dolasetron isomers that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Preparation of cis-7-ethoxycarbonyl-9-(ethoxycarbonylmethyl)-9-azabicyclo-[3,3,1]nonan-3-ol
[0088] Put 100g of 7-ethoxycarbonyl-9-(ethoxycarbonylmethyl)-9-azabicyclo-[3,3,1]non-3-one, 500ml of tetrahydrofuran and 10g of Raney nickel into the autoclave , feed hydrogen to a pressure of 0.1Mpa; slowly heat up to 90-100°C, adjust the hydrogen pressure to 0.5-0.6Mpa, stir until the reaction is complete, cool to room temperature, and filter the reaction solution through diatomaceous earth to remove Raney nickel , after the obtained filtrate was concentrated and dried under reduced pressure, the obtained brown-yellow oil was purified through a silica gel column (980 g of 200-300 mesh silica gel, packed with petroleum ether, washed with ethyl acetate / petroleum ether (1 / 10-1 / 5) deagent), to obtain light yellow oil 12g.
Embodiment 2
[0089] Example 2 Preparation of cis-7-ethoxycarbonyl-9-(ethoxycarbonylmethyl)-9-azabicyclo-[3,3,1]nonan-3-ol
[0090] Dissolve 100g of 7-ethoxycarbonyl-9-(ethoxycarbonylmethyl)-9-azabicyclo-[3,3,1]nonan-3-one with 500ml of absolute ethanol and add 12.5g in batches Sodium borohydride, heating to reflux, stirring, until the reaction is complete, add concentrated hydrochloric acid dropwise, adjust the pH of the reaction solution to 7~8, recover ethanol under reduced pressure, add water to dissolve the obtained concentrate after cooling, and extract to the water phase with ethyl acetate There was no product, the ethyl acetate layers were combined and dried with anhydrous sodium sulfate, anhydrous sodium sulfate was filtered off, the filtrate was concentrated to dryness under reduced pressure, and the obtained concentrate was purified on a silica gel column (950 g of 200-300 mesh silica gel, packed in petroleum ether, washed The deagent is ethyl acetate / petroleum ether (1 / 10~1 / 5)...
Embodiment 3
[0091] Example 3 Preparation of cis-7-ethoxycarbonyl-9-(ethoxycarbonylmethyl)-9-azabicyclo[3,3,1]nonan-3-ol-2-tetrahydropyranyl ether
[0092] Dissolve 20 g of cis-7-ethoxycarbonyl-9-(ethoxycarbonylmethyl)-9-azabicyclo-[3,3,1]nonan-3-ol in 100 ml of dichloromethane, cool in an ice bath, Slowly add 6g of methanesulfonic acid dropwise, then add 6g of 3,4-dihydropyran dropwise, stir until the reaction is complete, transfer the reaction solution into 200g of 40% potassium carbonate solution, extract with ethyl acetate, take the ethyl acetate layer It was dried with anhydrous sodium sulfate, filtered to remove anhydrous sodium sulfate, and the filtrate was concentrated to dryness under reduced pressure to obtain 24 g of brown-yellow oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com