A related substance of telamycin, its preparation method and application
A technology of telamycin and related substances, which is applied in the field of telamycin-related substances, and can solve the problems of lack of separation and detection methods for telamycin-related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] 5mg of telamycin API was dissolved in 1mL of acetonitrile, and the injection volume was 20μL.
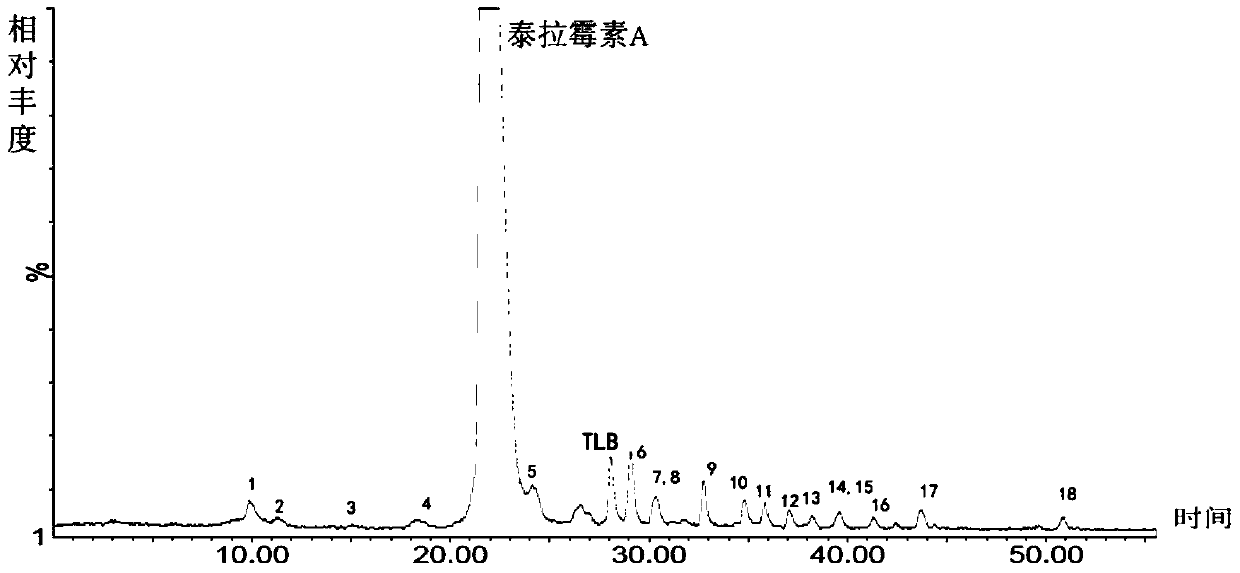
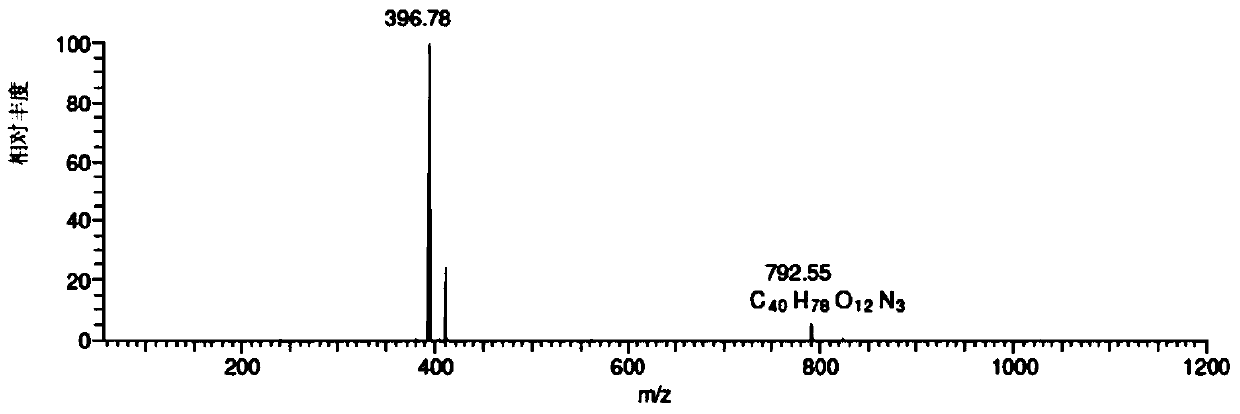
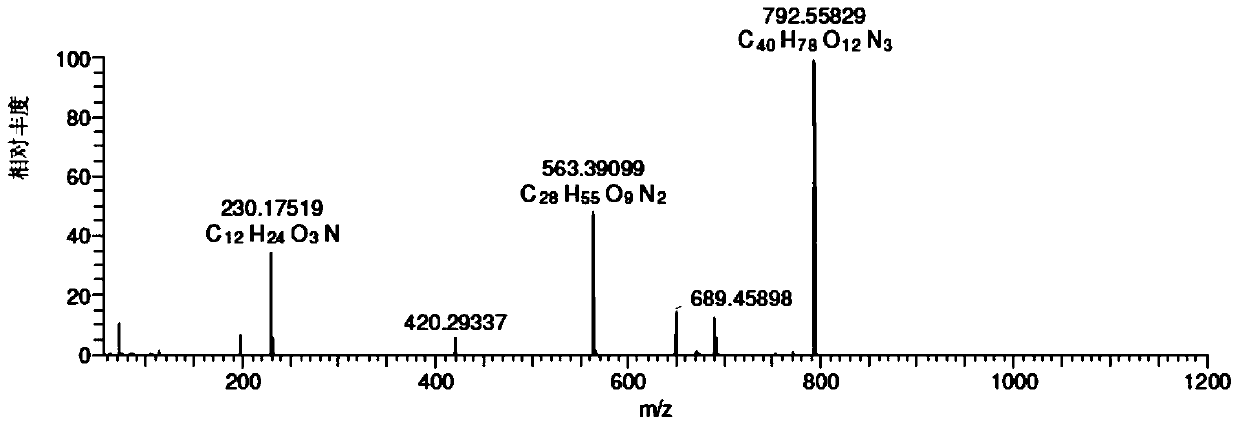
[0095] Chromatographic parameters: Xbridge TM C 18 (250*4.6mm, 5μm) column, mobile phase A: 0.35% formic acid aqueous solution (ammonia water to adjust the pH value to 7.66), mobile phase B: methanol:acetonitrile=45:25, gradient elution 0→15min, A:B=65 :35; 15→40min, A:B=65:35→30:70; 40→55min, A:B=30:70, UV absorption wavelength 205nm, column temperature 35℃, flow rate 1.0ml / min, HPLC fraction After passing through the UV detector, it enters MS detection with a 3:1 split. mass spectrometric total ion current as figure 1 Shown, each impurity is as shown in table 1. When the salt concentration of the mobile phase was 0.35%, the retention time of the main peak was appropriate and the peak shape was good. Related substances can be well separated from the main peak. It can be seen from the figure that the method of the present invention can be used to detect telamycin and i...
Embodiment 2
[0105] 5mg of telamycin API was dissolved in 1mL of acetonitrile, and the injection volume was 20μL.
[0106] Chromatographic parameters: Xbridge TM C 18 (250*4.6mm, 5μm) column, mobile phase A: 0.3% formic acid aqueous solution (ammonia water to adjust the pH value to 7.66), mobile phase B: methanol:acetonitrile=45:25, gradient elution 0→15min, A:B=65 :35; 15→40min, A:B=65:35→30:70; 40→55min, A:B=30:70, UV absorption wavelength 205nm, column temperature 35℃, flow rate 1.0ml / min, HPLC fraction After passing through the UV detector, it enters MS detection with a 3:1 split. mass spectrometric total ion current as Image 6 shown. When the salt concentration of the mobile phase was 0.3%, the retention time of the main peak was appropriate and the peak shape was good. Related substances can be well separated from the main peak.
Embodiment 3
[0108] 5mg of telamycin API was dissolved in 1mL of acetonitrile, and the injection volume was 20μL.
[0109] Chromatographic parameters: Xbridge TM C 18 (250*4.6mm, 5μm) column, mobile phase A: 0.4% formic acid aqueous solution (ammonia water adjusts the pH value to 7.66), mobile phase B: methanol:acetonitrile=45:25, gradient elution 0→15min, A:B=65 :35; 15→40min, A:B=65:35→30:70; 40→55min, A:B=30:70, UV absorption wavelength 205nm, column temperature 35℃, flow rate 1.0ml / min, HPLC fraction After passing through the UV detector, it enters MS detection with a 3:1 split. mass spectrometric total ion current as Figure 7 shown. When the salt concentration of the mobile phase was 0.4%, the retention time of the main peak was appropriate and the peak shape was good. Related substances can be well separated from the main peak.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com