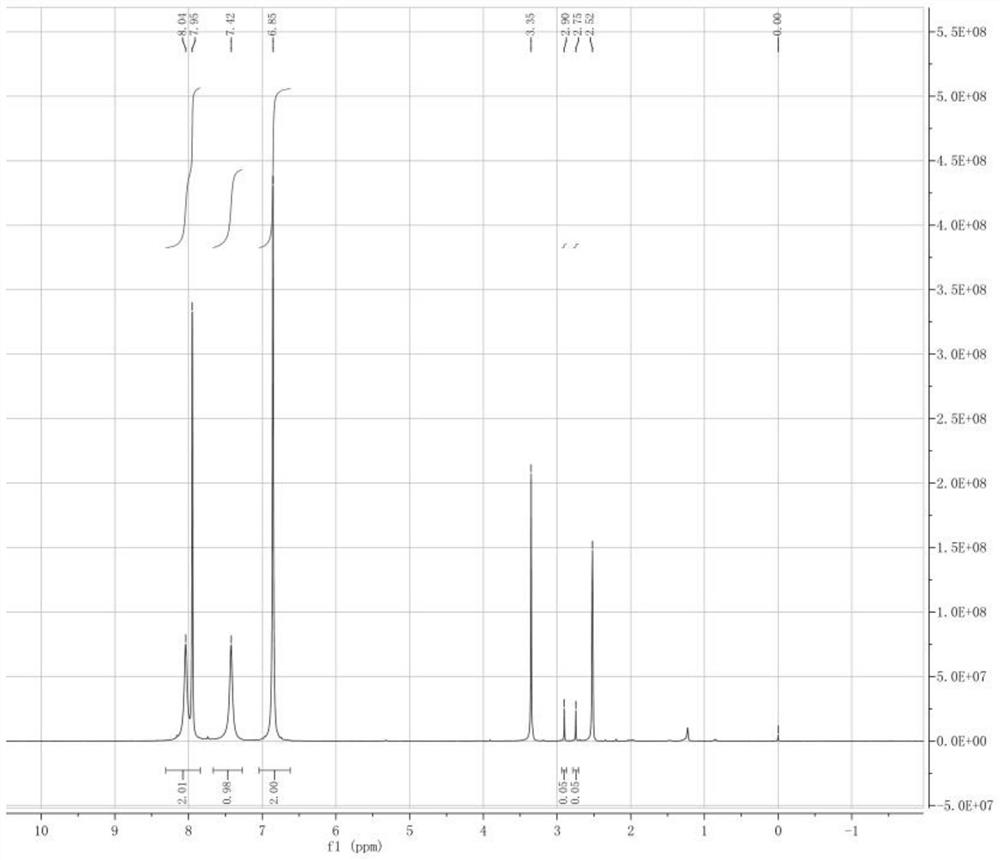
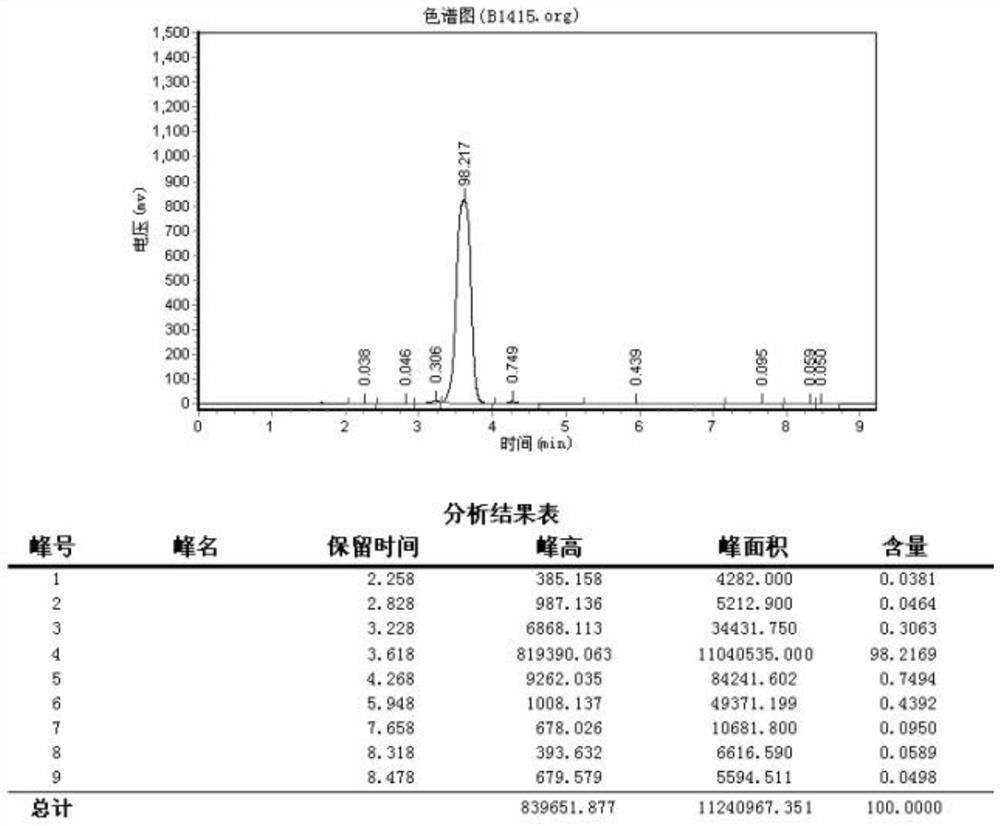
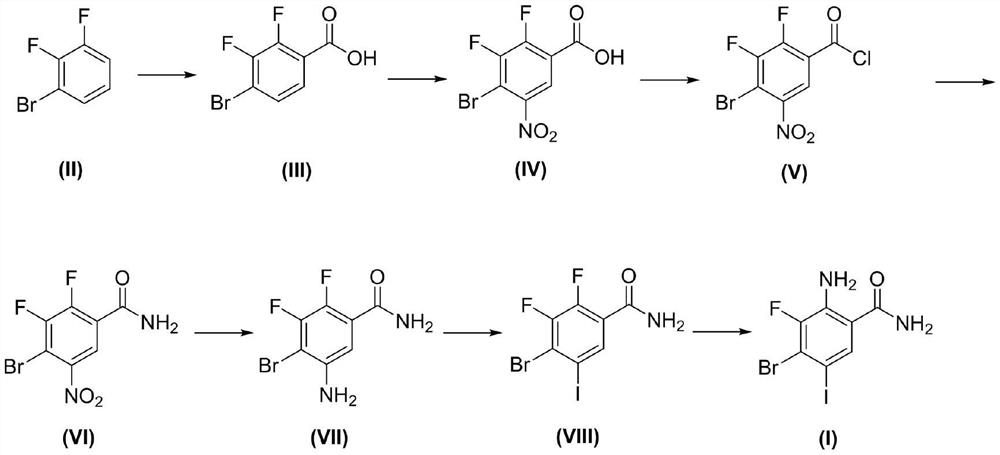
Preparation method of 2-amino-4-bromo-3-fluoro-5-iodobenzamide
A technology of iodobenzamide and amino group, which is applied in the preparation of carboxylic acid amides, nitro compounds, organic compounds, etc., can solve the problem of low yield of iodobenzamide, 2-fluoro-3-bromonitrobenzene Expensive, unfavorable industrial scale-up and other problems, to achieve the effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]A preparation method of 2-amino-4-bromo-3-fluoro-5-iodobenzamide, the preparation method comprising the following steps:
[0032](1) Synthesis of compound III
[0033]Add 193g (1mol) of 2,3-difluorobromobenzene, 1000ml of tetrahydrofuran to the reaction flask, and add 500ml of 2.5M butyllithium dropwise to -78°C. After the dropping, stir for 30min, and let carbon dioxide flow through it. Keep the temperature for 1 hour. The reaction was followed by HPLC until the 2,3-difluorobromobenzene reaction was completed; 1000ml of water was added to quench the reaction, 1000ml of ethyl acetate was added for layering, the aqueous layer was adjusted to pH=3, a solid was precipitated, filtered and dried to obtain 222g of compound III Molar yield: 93.6%.
[0034](2) Synthesis of compound IV
[0035]Add 200g (843.9mmol) of compound III, 1000ml of 98% concentrated sulfuric acid to the reaction flask, add 118.7g (1266mmol) of 68% concentrated nitric acid dropwise to 0°C, drop at 0°C and stir for 6h, and H...
Embodiment 2
[0049]A preparation method of 2-amino-4-bromo-3-fluoro-5-iodobenzamide, the preparation method comprising the following steps:
[0050](1) Synthesis of compound III
[0051]Add 193g (1mol) of 2,3-difluorobromobenzene, 1000ml of tetrahydrofuran to the reaction flask, add 400ml of 2.5M butyllithium dropwise to -78°C, and stir for 30min after dropping, then let it react with carbon dioxide at this temperature for 2 hours. The reaction was followed by HPLC until the 2,3-difluorobromobenzene reaction was complete; 1000ml of water was added to quench the reaction, 1000ml of ethyl acetate was separated into layers, the aqueous layer was adjusted to pH=3, a solid was precipitated, filtered and dried to obtain 201.8g of compound III , The molar yield: 85.1%.
[0052](2) Synthesis of compound IV
[0053]Add 200g (843.9mmol) of compound III, 1000ml of concentrated sulfuric acid to the reaction flask, add 98.9g (1055mmol) of 68% concentrated nitric acid dropwise to 30°C, slowly stir at 30°C for 2h, and HPL...
Embodiment 3
[0065]A preparation method of 2-amino-4-bromo-3-fluoro-5-iodobenzamide, the preparation method comprising the following steps:
[0066](1) Synthesis of compound III
[0067]Add 193g (1mol) of 2,3-difluorobromobenzene, 1000ml of tetrahydrofuran to the reaction flask, add 440ml of 2.5M butyllithium dropwise to -78°C, and stir for 30min after dropping, and then let it react at this temperature for 1.5 hours. The reaction was followed by HPLC until the 2,3-difluorobromobenzene reaction was complete; 1000ml of water was added to quench the reaction, 1000ml of ethyl acetate layered, the aqueous layer was adjusted to pH=3, a solid was precipitated, filtered and dried to obtain 211.4g of compound III , The molar yield: 89.1%.
[0068](2) Synthesis of compound IV
[0069]Add 200g (843.9mmol) of compound III, 1000ml of concentrated sulfuric acid to the reaction flask, drop 79.2g (843.9mmol) of 68% concentrated nitric acid, drop the temperature to 15°C, slowly warm to 15°C and stir for 4h, HPLC track the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com